SOLUTAB A
SOLUTAB®
®Registered Trademark(s) of Roquette Frères
- Wood source
10% max water content
Applications
- 1. Solid Forms - Tablets
- Swallowable Tablets
- Orally Dispersible Tablets
- 2. Solid Forms - Capsules
- Hard Capsules Fill
- 5. Other Solid Forms
- Granules and Pellets
Functional properties
- Formulations Aids
- Disintegrants & Super Disintegrants
Physical and chemical properties
- Health & Nutritional Benefits
- Sugar-free
- General Properties
- Label-friendly
- Multicompendial
Documents
Product Specification Sheet
Name
Region
Size
Download
SOLUTAB® A
476,74 Ko
Region :
476,74 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
SOLUTAB® A
Oceania, AU
EN
270,47 Ko
Region : Oceania, AU
EN
270,47 Ko
SOLUTAB® A
Europe, BE
EN
502,50 Ko
Region : Europe, BE
EN
502,50 Ko
SOLUTAB® A
Americas, CA
EN
284,55 Ko
Region : Americas, CA
EN
284,55 Ko
SOLUTAB® A
Europe, CH
EN
502,90 Ko
Region : Europe, CH
EN
502,90 Ko
SOLUTAB® A
Europe, CY
EN
499,73 Ko
Region : Europe, CY
EN
499,73 Ko
SOLUTAB® A
Asia, CN
EN
324,30 Ko
Region : Asia, CN
EN
324,30 Ko
SOLUTAB® A
Europe, DE
EN
506,83 Ko
Region : Europe, DE
EN
506,83 Ko
SOLUTAB® A
Europe, FR
EN
502,85 Ko
Region : Europe, FR
EN
502,85 Ko
SOLUTAB® A
Europe, AT
DE
517,34 Ko
Region : Europe, AT
DE
517,34 Ko
SOLUTAB® A
Europe, BE
DE
517,39 Ko
Region : Europe, BE
DE
517,39 Ko
SOLUTAB® A
Europe, BE
FR
616,24 Ko
Region : Europe, BE
FR
616,24 Ko
SOLUTAB® A
Europe, BE
NL
512,07 Ko
Region : Europe, BE
NL
512,07 Ko
SOLUTAB® A
Europe, BG
BG
660,46 Ko
Region : Europe, BG
BG
660,46 Ko
SOLUTAB® A
Americas, BR
PT
534,03 Ko
Region : Americas, BR
PT
534,03 Ko
SOLUTAB® A
Americas, CA
FR
396,67 Ko
Region : Americas, CA
FR
396,67 Ko
SOLUTAB® A
Europe, CH
DE
517,65 Ko
Region : Europe, CH
DE
517,65 Ko
SOLUTAB® A
Europe, CH
FR
616,93 Ko
Region : Europe, CH
FR
616,93 Ko
SOLUTAB® A
Europe, CH
IT
603,94 Ko
Region : Europe, CH
IT
603,94 Ko
SOLUTAB® A
Europe, CY
EL
651,83 Ko
Region : Europe, CY
EL
651,83 Ko
SOLUTAB® A
Europe, CY
TR
652,94 Ko
Region : Europe, CY
TR
652,94 Ko
SOLUTAB® A
Asia, CN
ZH
645,71 Ko
Region : Asia, CN
ZH
645,71 Ko
SOLUTAB® A
Europe, DE
DE
519,25 Ko
Region : Europe, DE
DE
519,25 Ko
SOLUTAB® A
Europe, CZ
CS
632,14 Ko
Region : Europe, CZ
CS
632,14 Ko
SOLUTAB® A
Europe, DK
DA
511,97 Ko
Region : Europe, DK
DA
511,97 Ko
SOLUTAB® A
Europe, ES
ES
520,64 Ko
Region : Europe, ES
ES
520,64 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Synonyms | Crosslinked carboxymethyl cellulose sodium |
|---|---|
| CAS number | 74811-65-7 |
| Physical form or apperance | White fine powder |
| Application | SOLUTAB® A croscarmellose sodium is a superdisintegrant for swallowable and orally dispersible tablets, hard capsules, blends, granules and pellets premix. It is flexible as it is adapted to wet and dry granulation or direct compression processes. |
| Source | Wood |
| Teste/Odor | Odorless |
| Morphology |
|
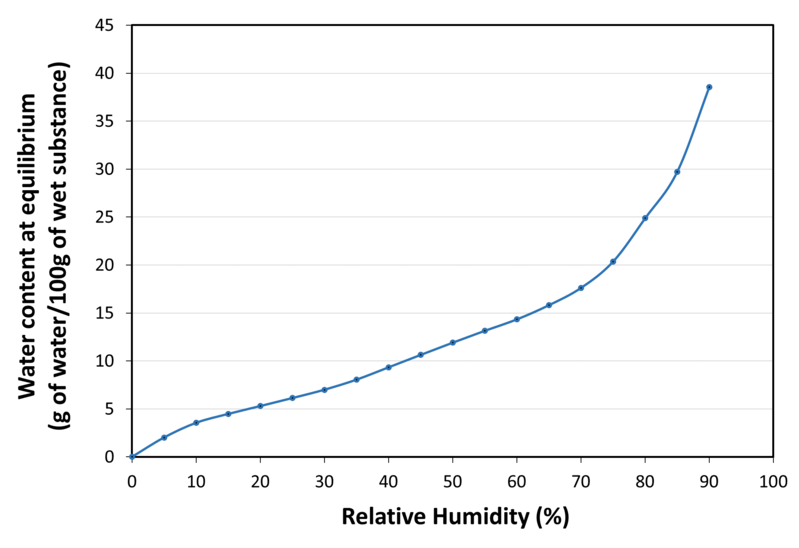
| Water sorption isotherm at 20°C |
|
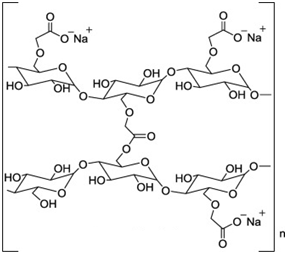
| Chemical Structure |
|
| Maximal Water content (LOD) | 10.00 |
| Minimum Molar substitution range | 0.60 |
| Maximum Molar substitution range | 0.85 |
| Solubility | Partially soluble in water; insoluble in alcohol, in ether, and in other organic solvents. |
| Average mean particle diameter | 60 |
| Bulk Density (g/ml) | 0.42 |
| Primary Mechanism of Action | Wicking, swelling |