SOLUTAB A-IP
SOLUTAB®
®Registered Trademark(s) of Roquette Frères
- Wood origin
6% max water content, Available as non-GMO
Applications
- 1. Solid Forms - Tablets
- Swallowable Tablets
- Orally Dispersible Tablets
- 2. Solid Forms - Capsules
- Hard Capsules Fill
- 5. Other Solid Forms
- Granules and Pellets
Functional properties
- Formulations Aids
- Disintegrants & Super Disintegrants
Physical and chemical properties
- Health & Nutritional Benefits
- Sugar-free
- General Properties
- Label-friendly
- Multicompendial
Documents
Product Specification Sheet
Name
Region
Size
Download
SOLUTAB® A-IP
479,39 Ko
Region :
479,39 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
SOLUTAB® A-IP
Oceania, AU
EN
268,63 Ko
Region : Oceania, AU
EN
268,63 Ko
SOLUTAB® A-IP
Europe, BE
EN
501,79 Ko
Region : Europe, BE
EN
501,79 Ko
SOLUTAB® A-IP
Americas, CA
EN
284,82 Ko
Region : Americas, CA
EN
284,82 Ko
SOLUTAB® A-IP
Europe, CH
EN
502,04 Ko
Region : Europe, CH
EN
502,04 Ko
SOLUTAB® A-IP
Europe, CY
EN
499,00 Ko
Region : Europe, CY
EN
499,00 Ko
SOLUTAB® A-IP
Asia, CN
EN
324,48 Ko
Region : Asia, CN
EN
324,48 Ko
SOLUTAB® A-IP
Europe, DE
EN
503,87 Ko
Region : Europe, DE
EN
503,87 Ko
SOLUTAB® A-IP
Europe, FR
EN
502,10 Ko
Region : Europe, FR
EN
502,10 Ko
SOLUTAB® A-IP
Europe, AT
DE
513,45 Ko
Region : Europe, AT
DE
513,45 Ko
SOLUTAB® A-IP
Europe, BE
DE
516,34 Ko
Region : Europe, BE
DE
516,34 Ko
SOLUTAB® A-IP
Europe, BE
FR
613,37 Ko
Region : Europe, BE
FR
613,37 Ko
SOLUTAB® A-IP
Europe, BE
NL
511,29 Ko
Region : Europe, BE
NL
511,29 Ko
SOLUTAB® A-IP
Europe, BG
BG
659,35 Ko
Region : Europe, BG
BG
659,35 Ko
SOLUTAB® A-IP
Americas, BR
PT
534,19 Ko
Region : Americas, BR
PT
534,19 Ko
SOLUTAB® A-IP
Americas, CA
FR
396,67 Ko
Region : Americas, CA
FR
396,67 Ko
SOLUTAB® A-IP
Europe, CH
DE
516,61 Ko
Region : Europe, CH
DE
516,61 Ko
SOLUTAB® A-IP
Europe, CH
FR
615,69 Ko
Region : Europe, CH
FR
615,69 Ko
SOLUTAB® A-IP
Europe, CH
IT
603,06 Ko
Region : Europe, CH
IT
603,06 Ko
SOLUTAB® A-IP
Europe, CY
EL
650,50 Ko
Region : Europe, CY
EL
650,50 Ko
SOLUTAB® A-IP
Europe, CY
TR
665,68 Ko
Region : Europe, CY
TR
665,68 Ko
SOLUTAB® A-IP
Asia, CN
ZH
612,86 Ko
Region : Asia, CN
ZH
612,86 Ko
SOLUTAB® A-IP
Europe, DE
DE
517,89 Ko
Region : Europe, DE
DE
517,89 Ko
SOLUTAB® A-IP
Europe, CZ
CS
630,73 Ko
Region : Europe, CZ
CS
630,73 Ko
SOLUTAB® A-IP
Europe, DK
DA
511,13 Ko
Region : Europe, DK
DA
511,13 Ko
SOLUTAB® A-IP
Europe, ES
ES
519,05 Ko
Region : Europe, ES
ES
519,05 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Synonyms | Crosslinked carboxymethyl cellulose sodium |
|---|---|
| CAS number | 74811-65-7 |
| Physical form or apperance | White fine powder |
| Application | SOLUTAB® A-IP croscarmellose sodium is a GMO-free superdisintegrant for swallowable and orally dispersible tablets, hard capsules, blends, granules and pellets premix. It is flexible as it is adapted to wet and dry granulation or direct compression processes. |
| Source | Wood, GMO-free |
| Teste/Odor | Odorless |
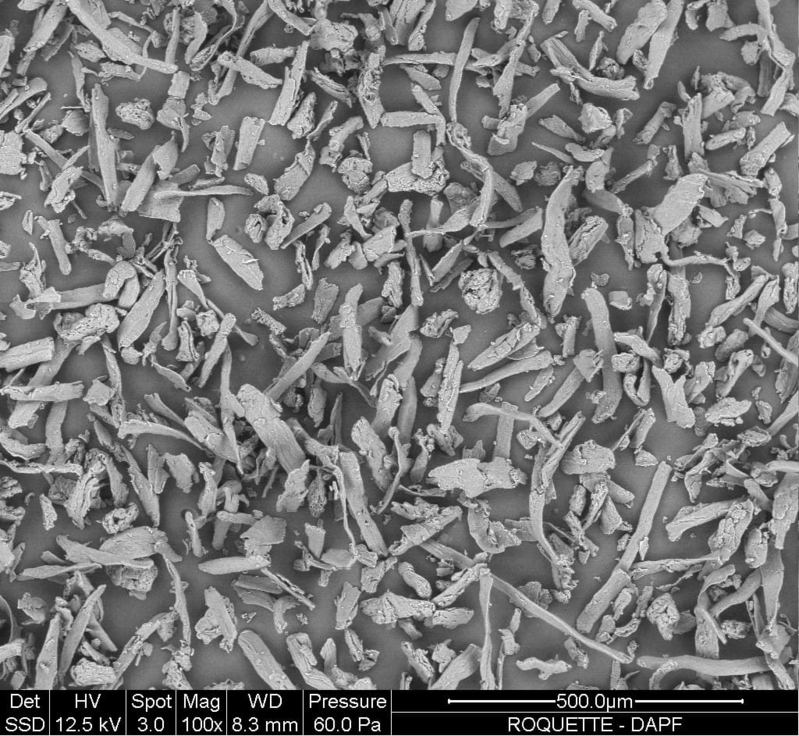
| Morphology |
|
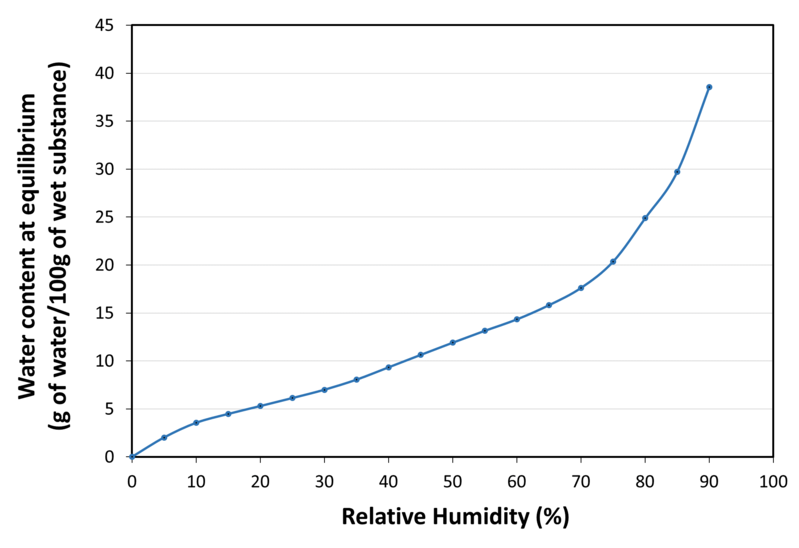
| Water sorption isotherm at 20°C |
|
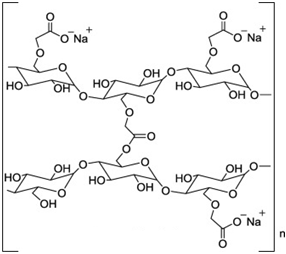
| Chemical Structure |
|
| Maximal Water content (LOD) | 6.00 |
| Minimum Molar substitution range | 0.60 |
| Maximum Molar substitution range | 0.85 |
| Solubility | Partially soluble in water; insoluble in alcohol, in ether, and in other organic solvents. |
| Average mean particle diameter | 60 |
| Bulk Density (g/ml) | 0.42 |
| Primary Mechanism of Action | Wicking, swelling |