MAIZE STARCH 400L-NF
- White color consistency
Oxidized maize starch
Applications
- 1. Solid Forms - Tablets
- Swallowable Tablets
- 2. Solid Forms - Capsules
- Hard Capsules Fill
- 5. Other Solid Forms
- Granules and Pellets
Functional properties
- Formulations Aids
- Disintegrants & Super Disintegrants
- Fillers and Binders for Direct Compression
- Fillers and Binders for Roller Compaction
Documents
Product Specification Sheet
Name
Region
Size
Download
400L-NF CORN STARCH
477,61 Ko
Region :
477,61 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
400L-NF CORN STARCH
Americas, CA
EN
270,95 Ko
Region : Americas, CA
EN
270,95 Ko
400L-NF CORN STARCH
Europe, CH
EN
492,56 Ko
Region : Europe, CH
EN
492,56 Ko
400L-NF CORN STARCH
Asia, CN
EN
312,05 Ko
Region : Asia, CN
EN
312,05 Ko
400L-NF CORN STARCH
Europe, FR
EN
492,48 Ko
Region : Europe, FR
EN
492,48 Ko
400L-NF CORN STARCH
Europe, GB
EN
355,83 Ko
Region : Europe, GB
EN
355,83 Ko
400L-NF CORN STARCH
Americas, Asia, Oceania, Africa
EN
257,20 Ko
Region : Americas, Asia, Oceania, Africa
EN
257,20 Ko
400L-NF CORN STARCH
Europe, IT
EN
495,80 Ko
Region : Europe, IT
EN
495,80 Ko
400L-NF CORN STARCH
Americas, US
EN
321,24 Ko
Region : Americas, US
EN
321,24 Ko
400L-NF CORN STARCH
Americas, CA
FR
382,64 Ko
Region : Americas, CA
FR
382,64 Ko
400L-NF CORN STARCH
Europe, CH
DE
504,29 Ko
Region : Europe, CH
DE
504,29 Ko
400L-NF CORN STARCH
Europe, CH
FR
603,50 Ko
Region : Europe, CH
FR
603,50 Ko
400L-NF CORN STARCH
Europe, CH
IT
592,87 Ko
Region : Europe, CH
IT
592,87 Ko
400L-NF CORN STARCH
Asia, CN
ZH
620,83 Ko
Region : Asia, CN
ZH
620,83 Ko
400L-NF CORN STARCH
Europe, ES
ES
506,85 Ko
Region : Europe, ES
ES
506,85 Ko
400L-NF CORN STARCH
Europe, FR
FR
603,31 Ko
Region : Europe, FR
FR
603,31 Ko
400L-NF CORN STARCH
Americas, Asia, Oceania, Africa
ES
269,30 Ko
Region : Americas, Asia, Oceania, Africa
ES
269,30 Ko
400L-NF CORN STARCH
Americas, Asia, Oceania, Africa
PT
274,54 Ko
Region : Americas, Asia, Oceania, Africa
PT
274,54 Ko
400L-NF CORN STARCH
Americas, Asia, Oceania, Africa
RU
398,05 Ko
Region : Americas, Asia, Oceania, Africa
RU
398,05 Ko
400L-NF CORN STARCH
Europe, IT
IT
595,20 Ko
Region : Europe, IT
IT
595,20 Ko
400L-NF CORN STARCH
Americas, MX
ES
273,72 Ko
Region : Americas, MX
ES
273,72 Ko
400L-NF CORN STARCH
Europe, SI
SL
592,84 Ko
Region : Europe, SI
SL
592,84 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Compliance | USP-NF |
|---|---|
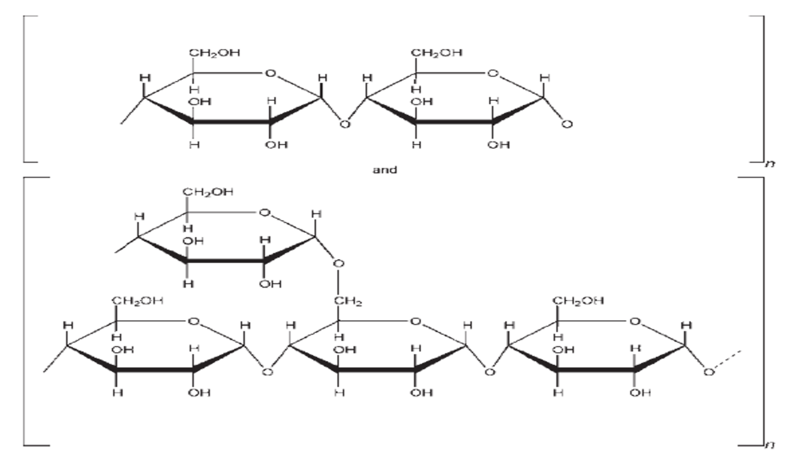
| Synonyms | Oxidized Starch |
| CAS number | 9005-25-8 |
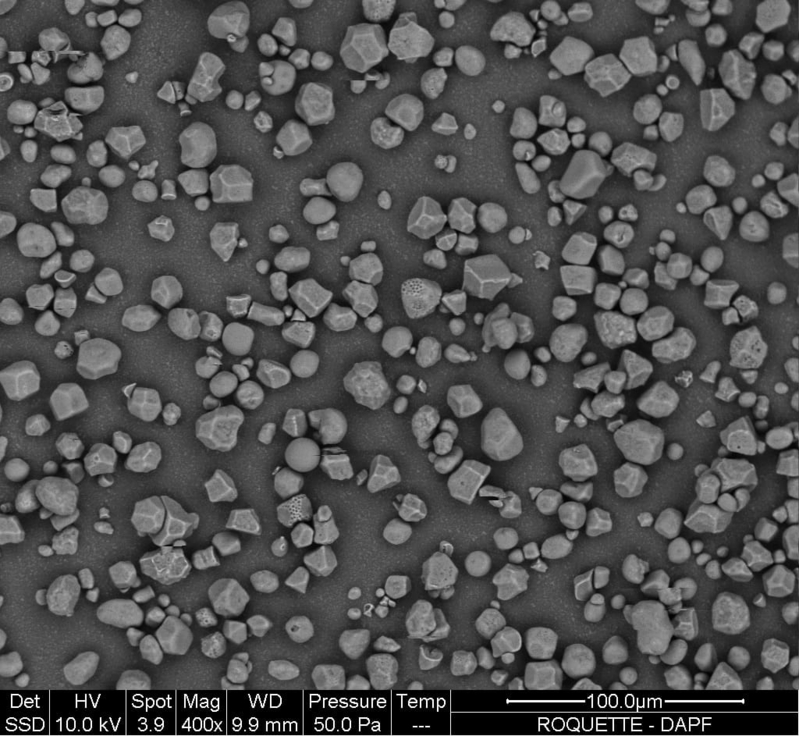
| Physical form or apperance | Very fine white powder that creaks when pressed between the fingers. |
| Application | 400L-NF Corn Starch is an oxidized native maize starch with disintegrant, filler and binder (once cooked) properties. It is used in a variety of pharmaceutical and nutraceutical dosages including swallowable tablets, hard capsules, blends, granules and pellet premix. Minimum recommended concentrations: 5% as binder and 10% as disintegrant. |
| Source | Maize |
| Teste/Odor | Odorless and tasteless |
| Morphology |
|
| Chemical Structure |
|
| Maximal Water content (LOD) | 13.50 |
| Solubility | Practically insoluble in cold water and in ethanol 96% |
| Gelatinization temperature | 73 |
| Average mean particle diameter | 16 |
| Powder Flowability (according to Ph.Eur. 2.9.16, 10mm outflow opening) | Not free-flowing |
| Bulk Density (g/ml) | 0.62 |
| Tapped Density (g/ml) | 0.83 |
| True Density (g/ml) | 1.48 |
| Angle of Repose (°) | 38 |
| Primary Mechanism of Action | Swelling |