Understanding the Superdisintegrant Performance of Croscarmellose Sodium from Different Suppliers – A Case Study with Different Tablet Matrices
Published February 11, 2026
Authors
Carin Siow
Pharmaceutical Application Scientist
Lesley Ooi
Senior Pharmaceutical Application Scient
Bing Xun Tan
GBU Pharma, Customer Technical Services Laboratory Manager, Singapore
Introduction
Croscarmellose sodium (CCS) is a superdisintegrant that promotes tablet disintegration by swelling and wicking mechanisms. When in contact with water, the omni-directional increase in the croscarmellose sodium particle size pushes the tablet apart. The wicking action draws water into the tablet, which helps to disrupt intermolecular forces between particles in the tablet.
It has been reported in the literature that factors such as the cellulose source or CCS particle size may result in differences in the attributes of CCS.1,2 For example, a larger particle size of CCS leads to enhanced swelling and faster disintegration because CCS forms a viscous layer upon contact with water, and a smaller particle size forms a more viscous layer because of enhanced interactions with water.2
Aside from the attributes of CCS, the performance of the superdisintegrant is largely dependent on the other components in the tablet formulation. Diluents can comprise up to 90% by weight of the dosage form, especially for a low-dose drug, to aid with product processability.2,3 As such, the nature and type of bulk material (either the excipient or active ingredient) could affect the tablet disintegration behavior and rate. This would then impact the superdisintegrant performance, especially if there is a comparison between different cellulose sources or suppliers. Mannitol and microcrystalline cellulose (MCC) are common diluents used in tablet formulations, but they exhibit different deformation characteristics and aqueous solubilities. The objective of the study was to evaluate the disintegrative performance of C
CS from different suppliers using two different tablet matrices (i.e., mannitol- or MCC-based), at different recommended use levels.
Materials and methods
SOLUTAB® A and SOLUTAB® EDP are two CCS grades that are offered by Roquette, which are of wood and cotton origin respectively. In this study, two other CCS marketed grades were selected for comparison, namely Excipient A (wood origin) and Excipient B (cotton origin). Typically, CCS concentrations of 0.5 to 5 % are recommended as further increase in the levels may result in the formation of a viscous gel layer, which may impede disintegration.3 PEARLITOL® 200 SD mannitol and MICROCEL® MC-102 MCC by Roquette were used to represent the water-soluble and insoluble tablet matrices in this study. 1 % magnesium stearate was included as a lubricant, and the proportion of mannitol and MCC were adjusted according to the various CCS concentrations. For all formulations, the proportions were determined based on a percentage weight per weight basis.
Tablets were compacted on the compaction simulator (STYL’One Evo, Medelpharm), with 10 mm round concave punches (D10R9). Tablets were compacted to similar thickness within the mannitol or MCC formulations. The tableting was simulating a FETTE P2090 Euro B equipment, at a speed of 25 rpm. The tablet thickness and hardness were evaluated using the hardness tester (PTB420, Pharma Test) and the disintegration time was characterized based on the USP method (PTZ Auto, Pharma Test). Complete disintegration is defined as that state in which no residue of the unit under the test remains on the screen of the apparatus. The disintegration behavior of the tablets was also captured via video imaging, by placing them into a petri dish containing water as the media without any mechanical agitation. One-way analysis of variance was performed at a significance level of p = 0.05 using a statistical analysis software (Minitab 18).
Results and discussion
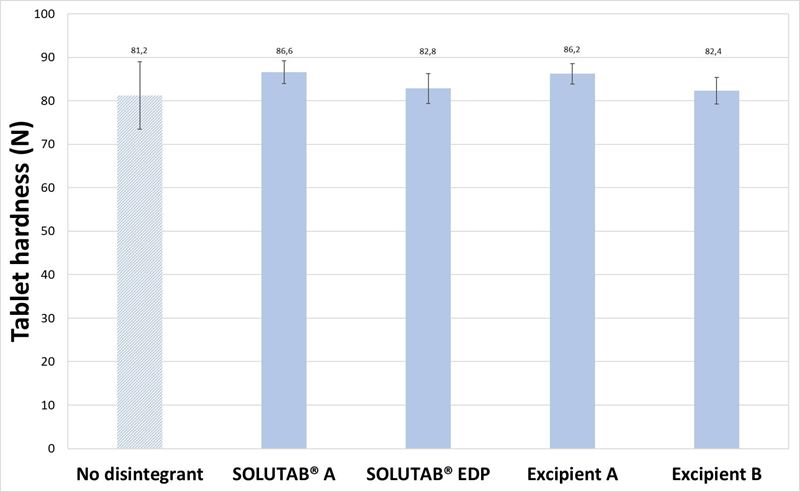
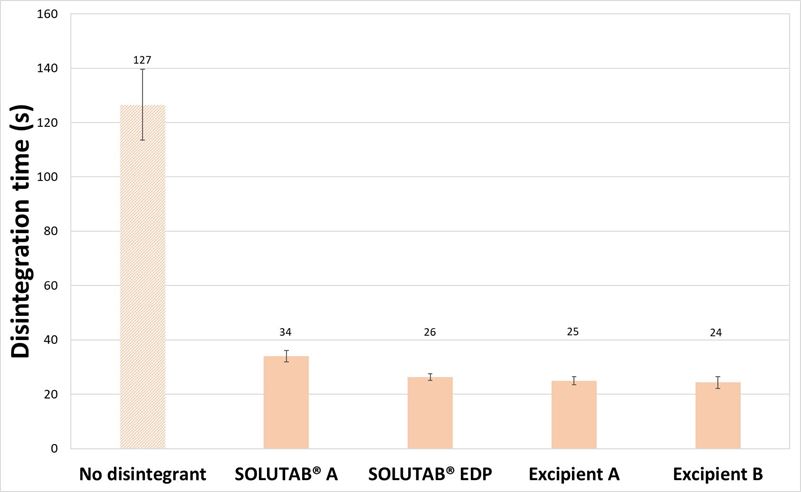
Tablets prepared with mannitol were tableted to a thickness of 5.24 ± 0.02 mm for both 1 % and 5 % CCS levels. For the tablets prepared with mannitol as the diluent and 1 %, w/w CCS, the resultant tablet hardness ranged between 81 to 87 N (Figure 1). The addition of 1 % croscarmellose sodium resulted in a significant decrease in disintegration time compared to the control formulation without disintegrant (126.5 s). The disintegration times of the tablets containing different croscarmellose sodium were comparable, under 35 s. The video images of the disintegrating tablets demonstrated that in the absence of CCS, the tablet surface was slowly eroding and dissolving in the media, which created entry points for water penetration and subsequently fracture points that broke up the tablet (Appendix I). With the addition of CCS at 1 %, the disintegration was started almost immediately when the tablet came into contact with the media, through the wicking property of CCS. Thereafter, the tablet was broken up quickly into smaller fragments that aided in reducing the overall tablet disintegration time.
(a)
(b)
Figure 1. (a) Hardness and (b) Disintegration time of mannitol-based tablets prepared without CCS (▨) or with 1 % CCS (■).
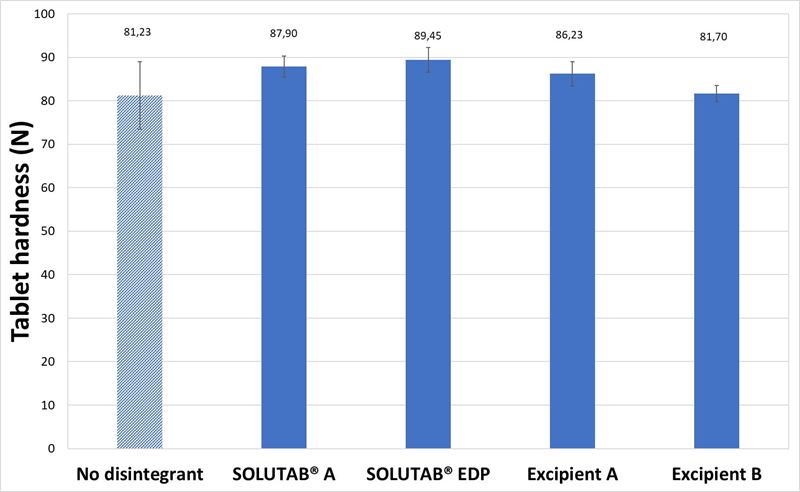
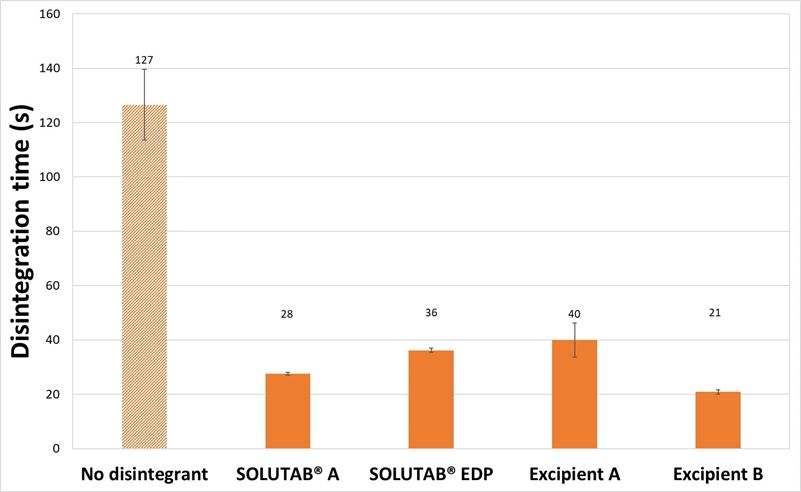
With 5 % CCS, the tablets were noted to be slightly harder compared to the mannitol tablets with 1 % CCS (Figure 2). Tablet disintegration time was still under or equal to 40 seconds. In terms of the disintegration behavior, swelling of the tablets were observed quickly for all the formulations, which was in agreement with the disintegration timings in the video (Appendix II). When adding 1% CCS, tablet disintegration times were already very short (less than 35 s), and it was expected that increasing to 5% CCS will provide further reduction to disintegration time. The use of high levels of CCS aimed to understand the impact of viscosity generated by CCS. The increase in viscous gel layer at the higher CCS concentration may lead to a slower disintegration rate. However, the changes in disintegration time between 1 % and 5 % CCS were very small. SOLUTAB® A (wood origin) and Excipient B (cotton origin) showed a decrease in disintegration time while SOLUTAB® EDP (cotton origin) and Excipient A (wood origin) showed an increase in tablet disintegration time when the CCS concentrations were increased respectively. Evidently, the origin of CCS (i.e., wood or cotton) was not the main reason that accounted for this difference, and it may be in part due to the CCS production process instead. From the videos captured, the swelling occurred faster for 5 % Excipient A formulations, but the mass was not disintegrating into fragments as that observed for 1 % (Appendix III).
(a)
(b)
Figure 2. (a) Hardness and (b) Disintegration time of mannitol-based tablets prepared without CCS (▨) or with 5 %, w/w CCS (■).
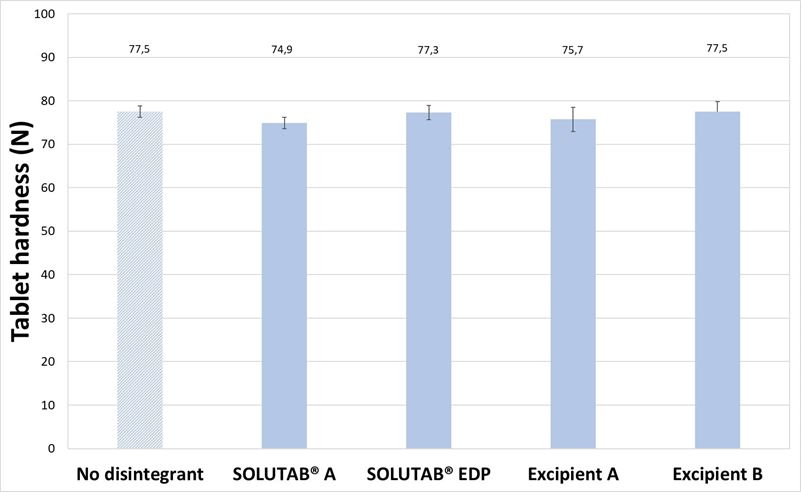
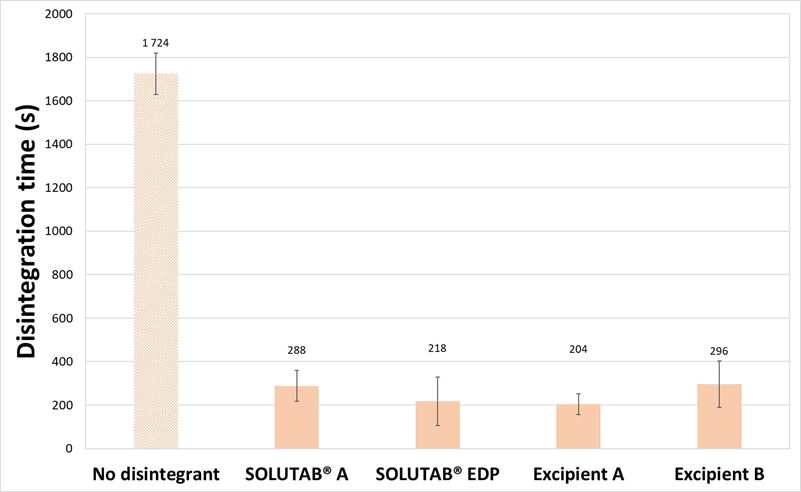
With MCC-based tablets, the impact of CCS addition on the disintegration time was apparent. For the MCC-based tablets without CCS and 1 % CCS, the tablets exhibited similar hardness (74.9 to 77.5 N; p > 0.05) as shown in Figure 3a. The thickness of MCC-based tablets without CCS was 5.23 ± 0.01 mm and tablets with 1 % CCS were 5.14 to 5.17 mm (p > 0.05). The addition of 1 %, w/w CCS resulted in a significant decrease in disintegration time from 1724 s (28.7 min) to around 200 to 300 s (Figure 3b). MCC is commonly employed as a filler-binder in the formulation, owing to its excellent compactability and binding properties.4 While MCC is known to exhibit self-disintegrating behavior through swelling of the MCC particles, it may not be sufficient on its own to promote tablet disintegration. On the other hand, with 1 % CCS, the wicking and swelling action of CCS helped to further break up the tablet into larger fragments (Appendix IV). Across the four grades of CCS evaluated, there was no significant difference in the tablet disintegration time (p > 0.05), which suggested that the performance of the CCS grades was similar.
(a)
(b)
Figure 3. (a) Hardness and (b) Disintegration time of MCC-based tablets prepared without CCS (▨) or with 1 % CCS (■).
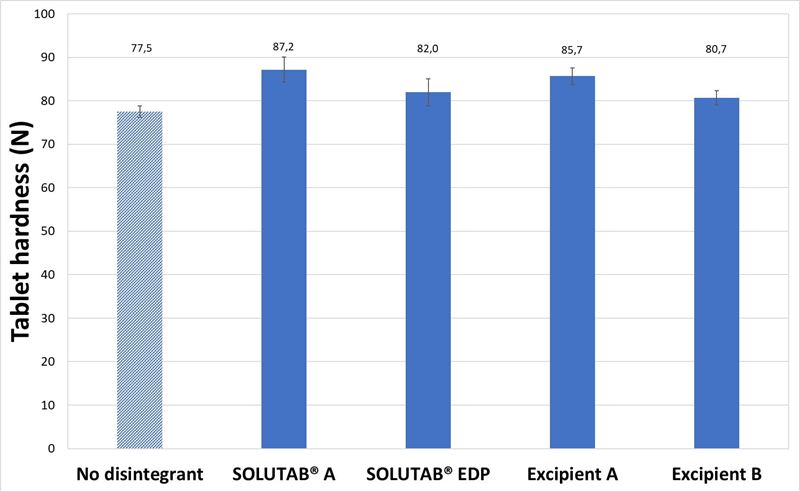
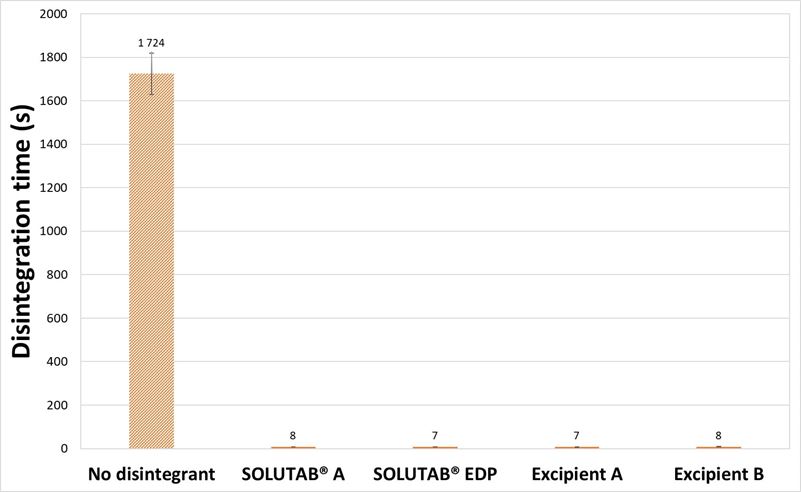
When 5 % CCS was incorporated in the formulation, the tablets were noted to be slightly harder, but exhibited relatively faster disintegration times of below 10 s. The increase in CCS concentration from 1 % to 5 %, for MCC-based tablets helped to further decrease the disintegration time by at least 90 %. The rapid disintegration behavior of the 5 % CCS was also observed from the video images (Appendix V).
(a)
(b)
Figure 4. (a) Hardness and (b) Disintegration time of MCC-based tablets prepared without CCS (▨) or with 5 % CCS (■). SD bars of 5 % CCS formulations are too small to be reflected.
Generally, the performance of CCS across the four grades were comparable. At the same CCS concentration, it was evident that the performance of CCS within the two matrices was different. The results of this study emphasized the importance of considering the tablet matrix when evaluating the performance of CCS of different grades and suppliers. The impact of CCS concentration was less pronounced for mannitol-based tablets, while 5 % CCS was more effective as shown in the MCC-based tablet formulations.
References
1.Koo OM, Editor. Pharmaceutical excipients: properties, functionality, and applications in research and industry. John Wiley & Sons, Inc.
2.Zarmpi P, Flanagan T, Meehan E, Mann J, Fotaki N. Biopharmaceutical aspects and implications of excipient variability in drug product performance. European Journal of Pharmaceutics and Biopharmaceutics. 2017 Feb 1;111:1-5.
https://doi.org/10.1016/j.ejpb.2016.11.004
3.Hiremath P, Nuguru K, Agrahari V. Material attributes and their impact on wet granulation process performance. In Handbook of pharmaceutical wet granulation 2019 Jan 1 (pp. 263-315). Academic Press.
https://doi.org/10.1016/B978-0-12-810460-6.00012-9
4.Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. International journal of pharmaceutics. 2014 Oct 1;473(1-2):64-72.
https://doi.org/10.1016/j.ijpharm.2014.06.055
Appendices
5.Appendix V. Videos of MCC-based tablets prepared without CCS or with 5 % CCS disintegrating in water
Disclaimer
MICROCEL® is a registered trademark in Benelux, Brazil, Canada, Chile, France, Germany, Italy, Mexico, the United Kingdom, and the United States of America and is pending in other countries or regions.
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.