Benefits of adding crystalline mannitol as filler in wet granulation
Published March 05, 2026
Authors
Philippe Lefèvre
Head of Global Pharma Applications Labs, Roquette
Introduction
Mannitol presents a high chemical stability and is the only excipient having two opposite properties: water solubility (18 g/100 ml at 20°C) and low hygroscopicity (pure mannitol powder does not adsorb water at 20°C up to 96% relative humidity). Therefore, it is considered compatible with almost all drugs, and it is the first excipient designed for APIs that face stability problems during formulation.
In wet granulation process, mannitol is partly solubilized by the aqueous granulation liquid. However, upon drying, mannitol recrystallizes quickly and totally in its initial beta crystalline form, improving the stability and properties consistency of granules.
Mannitol is thus used as a diluent improving the stability of the final medicine. It also has a positive impact on the properties of granules and tablets.
Experimental strategy
In this study, the impact of adding mannitol in a wet granulation process was evaluated by comparing formulations with and without mannitol and measuring the characteristics of granules and tablets.
Acetaminophen was used as a model API. Two distinct natures of binders were used. LYCATAB® PGS is a pregelatinized starch which properties are perfectly adapted to high shear wet granulation process and GLUCIDEX® 12, a maltodextrin with a high solubility facilitating the granulation. Sodium starch glycolate GLYCOLYS® LV was used as superdisintegrant.
GLYCOLYS® LV has been specifically developed for high shear granulation. After the addition of water, part of the grains of a standard sodium starch glycolate grade will absorb water and start to swell. They then become sensitive to high shear, leading to damaged grains and loss of their disintegrating properties. At this stage, standard sodium starch glycolate will act more as binder. GLYCOLYS® LV grains are strengthened to support high shear wet granulation.
Materials and methods
Materials
Acetaminophen with particle size distribution from 50µm to 250µm,
PEARLITOL® 160C crystalline mannitol powder (Roquette Frères, France),
LYCATAB® PGS pregelatinized maize starch (Roquette Frères, France),
GLUCIDEX® 12 maltodextrin (Roquette Frères, France),
GLYCOLYS® LV sodium starch glycolate (Roquette Frères, France),
Vegetal magnesium stearate, Demineralized water.
Formulation
Table 1. Powder blends composition used for high shear wet granulation
% weight | PGS/MAN0 | PGS/MAN20 | GLU/MAN0 | GLU/MAN20 |
| ACETAMINOPHEN | 87.5 | 67.5 | 87.5 | 67.5 |
| PEARLITOL® 160C | 20 | 20 | ||
| LYCATAB® PGS | 7.5 | 7.5 | ||
| GLUCIDEX® 12 |
| 7.5 | 7.5 | |
GLYCOLYS® LV | 5.0 | 5.0 | 5.0 | 5.0 |
Granulation trials
500 g of powder blend, acetaminophen, mannitol, binder, sodium starch glycolate were mixed for two minutes in a high shear granulator Bohle BMG (Bohle, Germany), (impeller at 250 rpm and chopper at 1500 rpm); 300 g of demineralized water was sprayed for 4 minutes and mixing continued for 4 additional minutes. Obtained granules were dried in an Aeromatic Strea-1TM (Aeromatic, UK) at 60°C during 30 min and calibrated with an Erweka oscillating calibrator (800µm) (Erweka, Germany). The granulation was repeated twice to have the granules quantity necessary for analysis and for tableting. Granules’ properties were measured following European Pharmacopeia methods.
Tableting trials
750 g of granules (99.0%) and magnesium stearate (1.0%) were mixed in a Turbula mixer (WAB, Switzerland) for 5 minutes. Tablets were made with Fette Exacta 21 single punch tablet press (Fette, Germany) equipped with D13 beveled flat punches at 4 compression forces (15, 20, 25, 30 kN). To maintain the acetaminophen content at 500mg per tablet, the weight of tablet differed: 578 mg without mannitol and 749 mg with mannitol.
Tablets’ evaluation
Weight, thickness, diameter and hardness were evaluated with an Erweka TBH30 GMD (Erweka, Germany). Disintegration times were measured with an Erweka ZT 3 (Erweka, Germany). Tablets’ friability was measured with an Erweka TAR220 (Erweka, Germany).
Results
The optimum amount of water for each formulation was determined using the evolution of the torque value of the main propellor. Whatever the formula composition, this water requirement remains similar (from 28 to 31 g of water for 100 g of powder, table 2). The addition of mannitol seems to have no impact on the quantity of water required for granulation.
Table 2. Ratio of water for optimal granulation and granules’ properties
Formulation | Water ratio (g/100g powder) | Loss on drying (%) | Water activity (-) | Bulk density (g/ml) | Hausner ratio (-) | Flow time (s) |
PGS/MAN0 | 29 | 1.2 | 0.37 | 0.488 | 1.27 | 9 |
PGS/MAN20 | 31 | 1.1 | 0.39 | 0.505 | 1.21 | 12 |
GLU/MAN0 | 28 | 1.2 | 0.43 | 0.543 | 1.24 | 9 |
GLU/MAN20 | 28 | 0.9 | 0.41 | 0.526 | 1.25 | 8 |
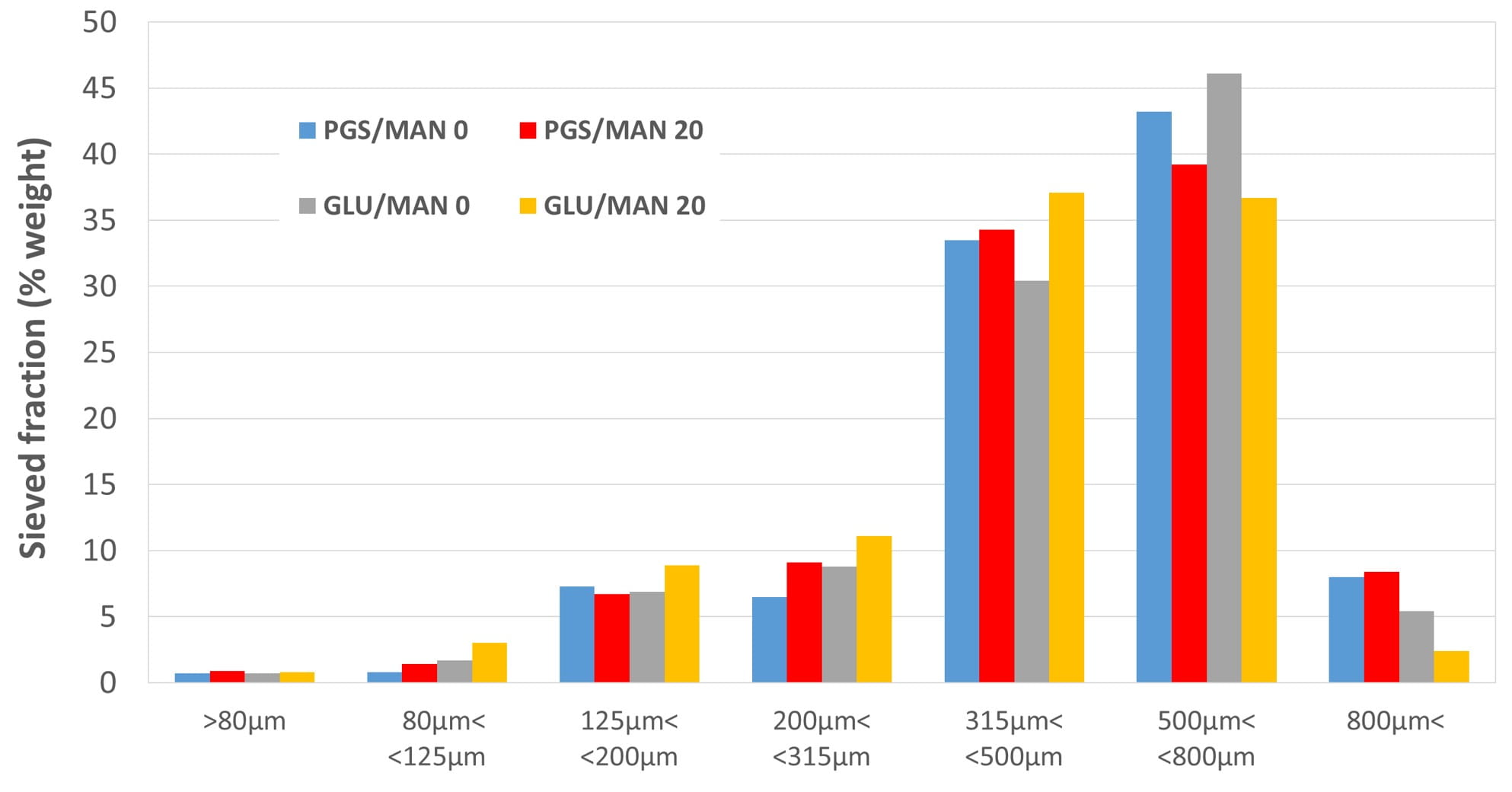
According to their physical characteristics and particle size distribution (table 2 and figure 1), all granules were considered to be suitable for being tableted.
Figure 1. Granules’ particle size distribution measured by sieving.
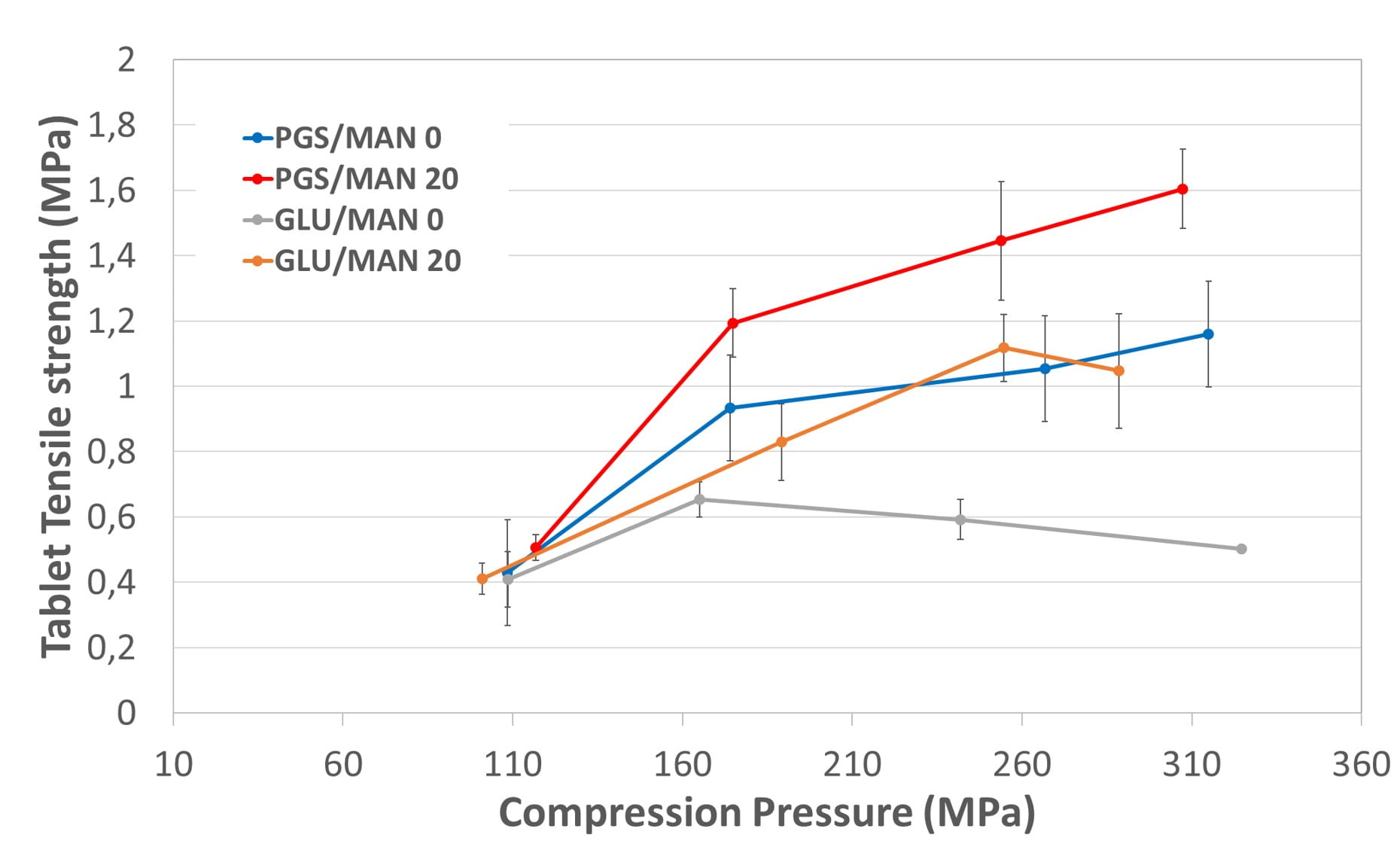
Figure 2 shows the impact of adding mannitol as filler on the compactibility of the granules. The slope of the tensile strength versus compression pressure was dramatically changed so that the difference of tensile strength increased greatly with the compression pressure; up to about 40% increase for LYCATAB® PGS and 90% increase for GLUCIDEX® 12 at the highest compression pressures. Without mannitol, tablet tensile strength stays below 1.2 MPa, which corresponds to a tablet hardness lower than 100 N while tensile strength higher than 1.2 up to 1.6 MPa (tablet hardness higher of 150 N) was accessible adding mannitol to LYCATAB® PGS formulation.
Figure 2. Tabletability of the granules.
All GLUCIDEX® 12 formulations gave tablets with high friability due to tablet capping during the test. On the contrary, tablets containing LYCATAB® PGS presented a low friability associated with a positive impact of the presence of mannitol. Without mannitol, tablets friability decreased from 1.2% to 0.8% with tensile strength increase from 0.43 to 1.16 MPa while with mannitol the decrease was from 0.7% to 0.4% for tensile strength increasing from 0.51 to 1.60 MPa. Mannitol thus presents here a clear effect on tablet friability decrease.
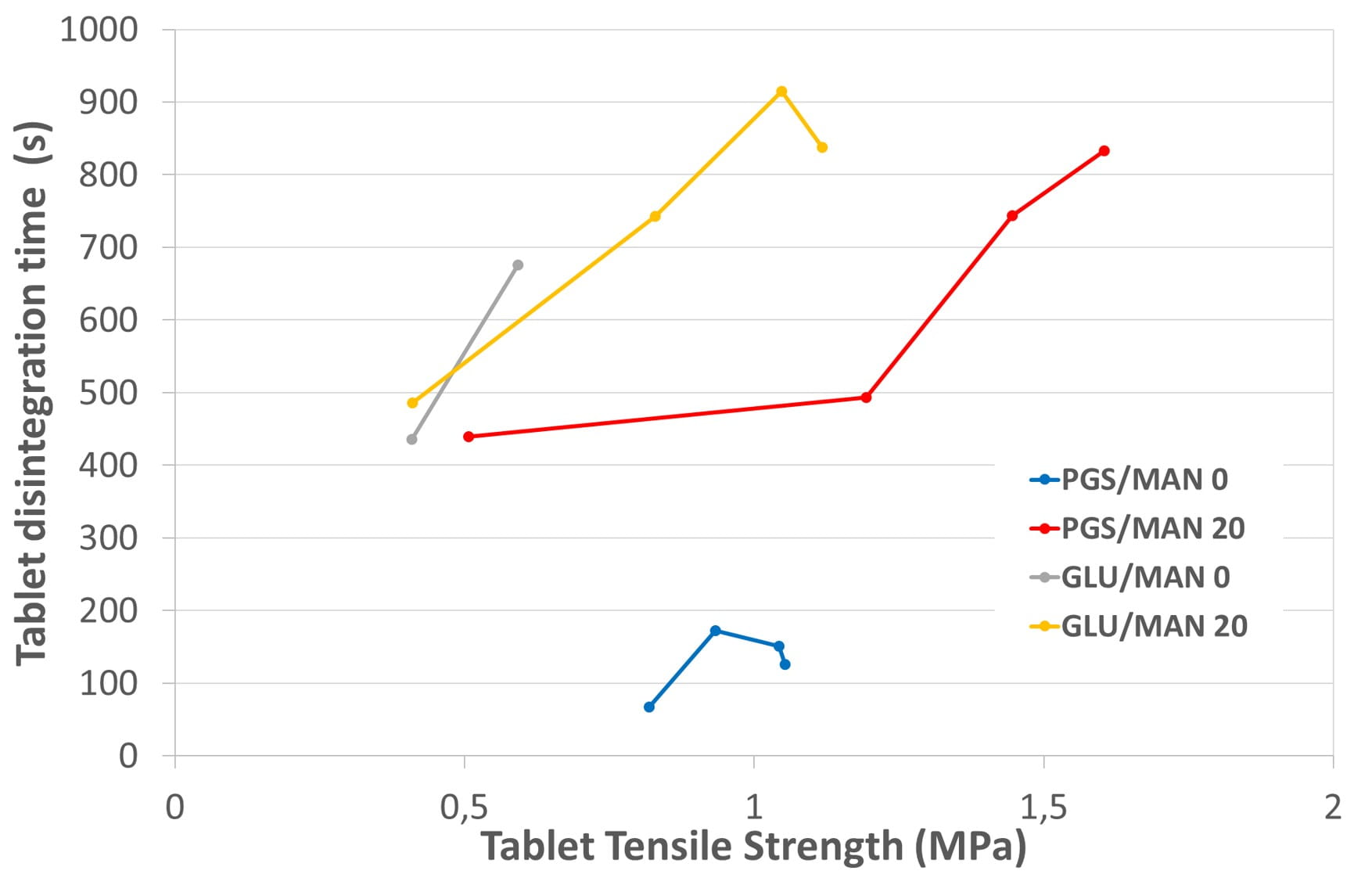
Figure 3. Tablets’ disintegration.
Finally, disintegration times were measured and are shown on figure 3.
With LYCATAB® PGS as binder, adding mannitol leads to the increase of tablet disintegration time. However, the maximum disintegration time measured here remains lower than 15 minutes corresponding to the standard expectation from the pharmacopeia.
It is presumed that this increase of disintegration time is due to the hydrophily of mannitol generating a competition with the superdisintegrant for water. At the same time, the viscosity increase due to the mannitol dissolution may also slow down the adsorption of water, even if mannitol is well known to have a limited impact on these two phenomena.
The mannitol-free tested formulations did not give access to acceptable tablets, whereas formulation PGS/MAN20 including 7.5% LYCATAB® PGS as binder and 20% mannitol as filler answered all the needs: easy high shear wet granulation process, granules with expected characteristics, notably free-flow and particle size, tablets with more than 100 N hardness and low friability (0.4%) done at about 25 kN compression force. The disintegration time of about 10 min could be decreased by an extra granular addition of superdisintegrant, as 2.5% GLYCOLYS LV.
Conclusion
Mannitol is commonly used in pharmaceutical tablets to improve API and tablet stability. To determine other potential impacts, high shear wet granulation was performed on four acetaminophen tablet formulations, two without mannitol and two with mannitol as filler.
Adding mannitol had no impact on the granulation process nor on the granule’s particle size or flow behavior. On the contrary, it improved substantially the tablet cohesion: higher tablet hardness and lower friability, while increasing the disintegration time. Finally, only the formulation containing mannitol PEARLITOL® 160C and pregelatinized maize starch LYCATAB® PGS, gave access to acetaminophen tablet formulation potentially conforming to the corresponding monograph.
References
- A. C. Santomaso, R. Baggio, F. Zorzi, G. Salviulo, N. Realdon, E. Franceschinis, Sugars with different thickening power in high shear granulation
- C.W. SYMECKO, C.T. RHODES, The Effect of Compaction Force and Type of Pregelatinized Starch on the Dissolution of Acetaminophen, Drug Development and Industrial Pharmacy, 23(3), 229-238 (1997)
® Registered trademark(s) of Roquette Frères. The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without any guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission. For questions about a product’s compliance with additional countries’ standards not listed above, please contact your local Roquette representative.