Enabling Stable and Directly Compressible Probiotic Tablet Formulation
Published March 06, 2026
Presented at the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, 18 - 21 March 2024, Vienna, Austria.
Authors
Carin Siow
Pharmaceutical Application Scientist
Lesley Ooi
Senior Pharmaceutical Application Scient
Kwan Hang Lam
Analytical Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Low Jeslyn
GBU Pharma, Assistant Research Scientist, Singapore
Bing Xun TAN
GBU Pharma, Customer Technical Services Laboratory Manager, Singapore
Introduction
The growing interest in probiotic supplements is driven by the favorable effects on intestinal microbiota and contributions to the health of the host environment, many of which are backed by scientific studies.1 Probiotic microorganism strains such as lactic acid bacteria and Bifidobacterium have a long history of safe use and confer various beneficial effects when ingested in appropriate concentrations. Chewable probiotic tablets are an attractive dosage form because of its convenience and the ease of administration and swallowability. However, the present challenge in probiotic tablet formulations is the maintenance of sufficient number of viable cells at the time of consumption.2
The ideal water activity (Aw) for probiotic microorganism survival is generally reported to be between 0.2 to 0.3.3 If the Aw of the microorganism is too low, the dehydrated state could cause the membrane lipids to be oxidized, leading to reduced viability. Conversely, if the Aw is too high, there is an increased risk of uncontrolled solubility and chemical reactions between organic compounds. As such, the selection of a low Aw excipient in a probiotic formulation is imperative for probiotic stability reasons. As a tableting excipient, it should exhibit good processability and confer a pleasant taste profile in the final formulation. PEARLITOL® ProTec co-processed mannitol-starch excipient was hence developed to meet these needs.
The objectives of this study were to characterize PEARLITOL® ProTec and evaluate the stability of probiotic formulations comprising of PEARLITOL® ProTec at the following stages: (i) post-tablet production and (ii) storage over 3 months.
Materials and Methods
I. Characterization of PEARLITOL® ProTec excipient
The morphology of PEARLITOL® ProTec (Roquette, France) was observed with the scanning electron microscope (JSM-IT200LV, JEOL). The water activity was measured (Aqualab 4TE Duo, Meter Group, USA) at 25 °C while the moisture content was determined by the oven drying method at 130 °C. The particle size distribution was evaluated on the laser sizer (Mastersizer 3000, Malvern Panalytical). The bulk and tapped density of PEARLITOL® ProTec was measured based on Method 1 as described in European Pharmacopoeia (EP) 2.9.34, where the Carr’s Index and Hausner ratio were determined. The hardness of the tablets obtained was measured with a hardness tester (TBH 425, ERWEKA).
II. Stability evaluation of probiotic tablet containing PEARLITOL® ProTec
Lactobacillus rhamnosus (THT, Belgium) was selected as the model probiotic strain in this study. The probiotic tablet formulation was composed of 24.75 % of Lactobacillus rhamnosus, 74.25 % of PEARLITOL® ProTec and 1 % magnesium stearate (MgSt; Roquette, France).
As control, tablets containing 99% of Lactobacillus rhamnosus powder and 1% of magnesium stearate were also produced.
The tablets were made as follows: biconvex caplets with a length of 19 mm, a width of 9.5 mm and a weight of about 1 g were prepared on the compaction simulator STYL’One Evo (MEDELPHARM, France), simulating a FETTE 2090 rotary press at a speed of 25 rpm (54000 tablets/h), using a pre-compression force of 1.0 to 1.3 kN. The tablets were packed in aluminum blister and Lactobacillus rhamnosus neat powder was packed in aluminum sachets. Both were stored in two different conditions: fridge and 25 °C/60 % relative humidity (RH). Tablets were characterized as follow: Aw, moisture content (halogen moisture analyzer (HC103, Mettler Toledo) at a drying temperature of 105 °C), hardness, disintegration time (EP 5.0 2.9.1.) using a disintegration tester (PTZ Auto, Pharma Test) and probiotic viability. Probiotic viability was also assessed on Lb. rhamnosus neat powder as reference. Those characterizations were carried out at pre-specified time points over three months. The viability of Lactobacillus rhamnosus was determined using the validated 3M petrifilm method using the LAB plate and incubated for 48 hours under 32 °C.
Results And Discussions
I. Characterization of PEARLITOL® ProTec excipient
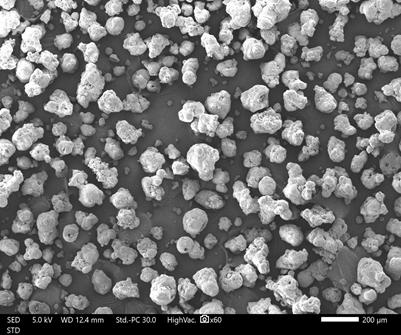
The appearance of PEARLITOL® ProTec is shown in Figure 1, and the measured physical properties are listed in Table 1. PEARLITOL® ProTec exhibited good flow properties based on the Carr’s Index and Hausner ratio. Furthermore, the low Aw and moisture content of PEARLITOL® ProTec are important considerations with regards to the choice of a suitable filler excipient to be incorporated with probiotics, to ensure stability of the formulation.
Figure 1. Appearance of PEARLITOL®ProTecunder (a) 60x and (b) 160x magnifications.
Table 1. Physical properties of PEARLITOL® ProTec
| Property | Measured average value (standard deviation) |
| Water activity | 0.122 ± 0.006 |
| Moisture content | 0.740 ± 0.068 % |
| Particle size distribution | |
| D10 | 74 ±1 µm |
| D50 | 126 ± 2 µm |
| D90 | 210 ± 7 µm |
| Bulk density | 0.487 ± 0.003 g/mL |
| Tapped density | 0.562 ± 0.005 g/mL |
| Carr’s Index | 13.3 ± 1.34 % |
| Hausner ratio | 1.15 ± 0.02 |
II. Stability evaluation of probiotic tablet containing PEARLITOL® ProTec
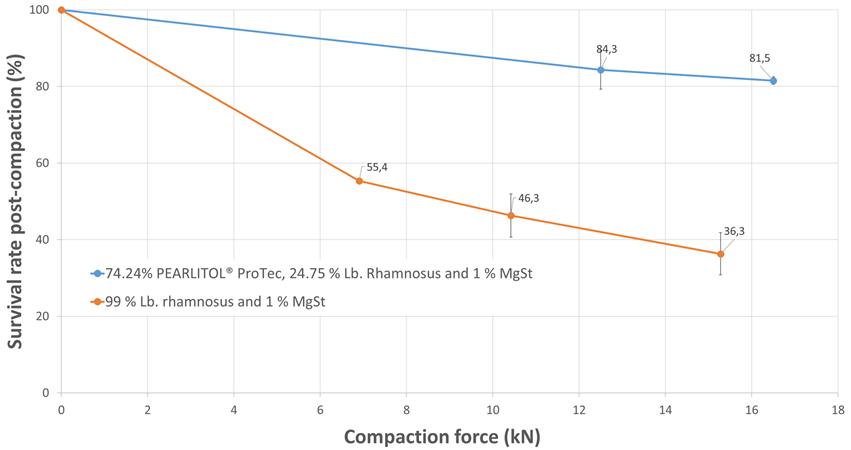
It is well understood that the compaction force has an impact on the viability of the probiotic, where a higher force typically results in a greater loss in viability. It has earlier been reported by Byl et al. that a certain level of elastic recovery is useful in safeguarding the viability of Lb. rhamnosus GG.2 Hence, the post-compaction viability was determined to understand the protective effect on the probiotic that could be conferred by PEARLITOL® ProTec during compaction. As shown in Figure 2, the protection conferred by PEARLITOL® ProTec was demonstrated even at higher compaction forces.
Figure 2. Survival rate of Lb. rhamnosus after compaction at different forces, with and without PEARLITOL® ProTec.
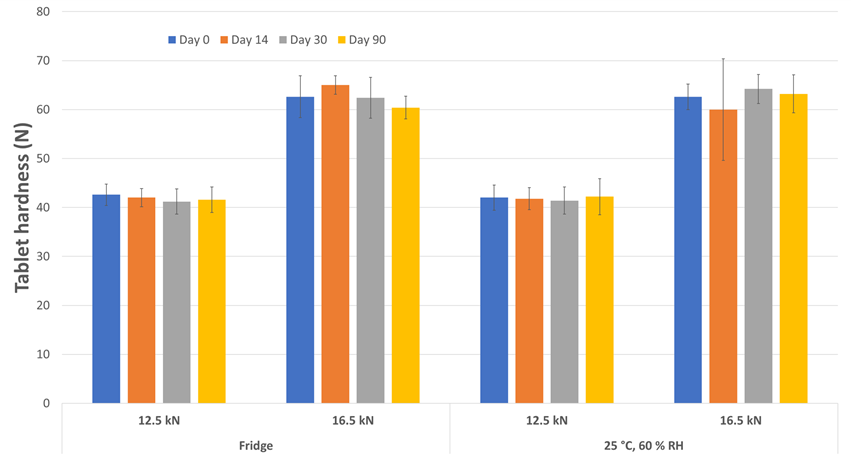
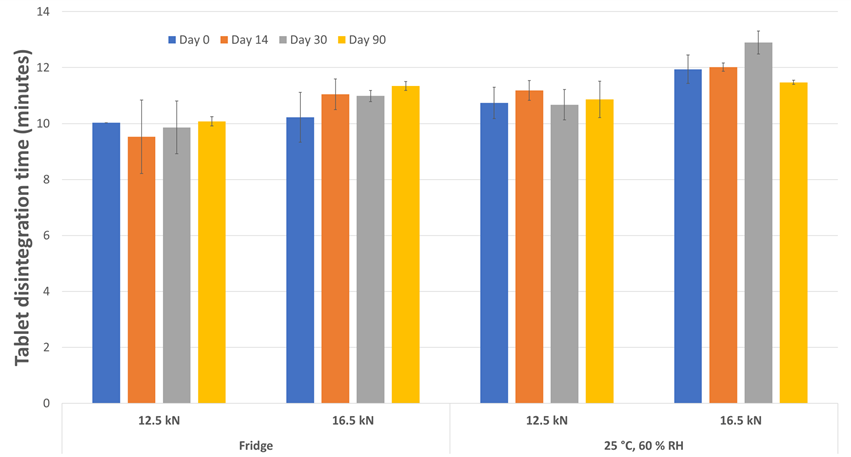
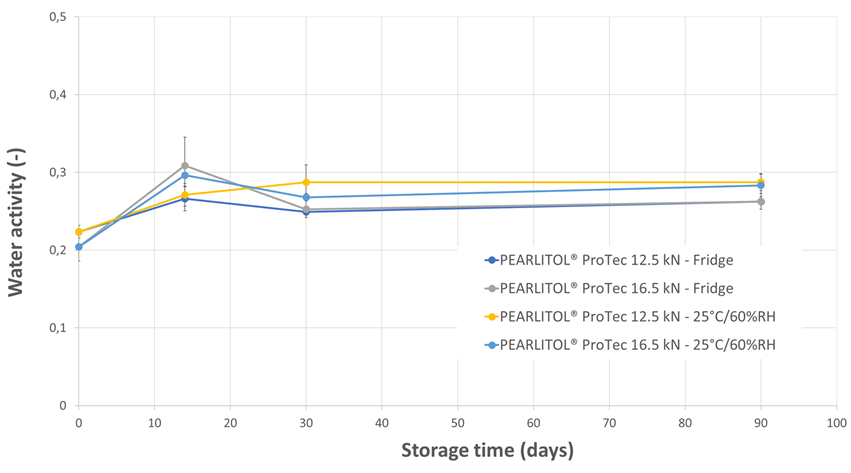
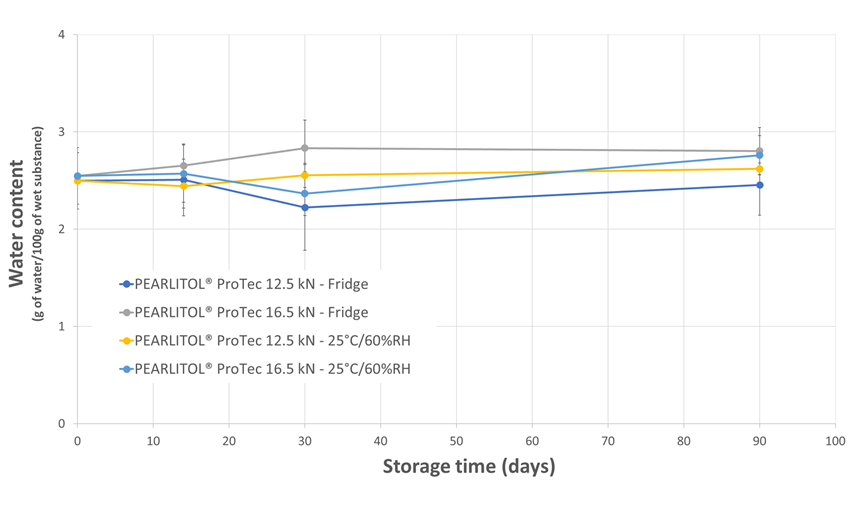
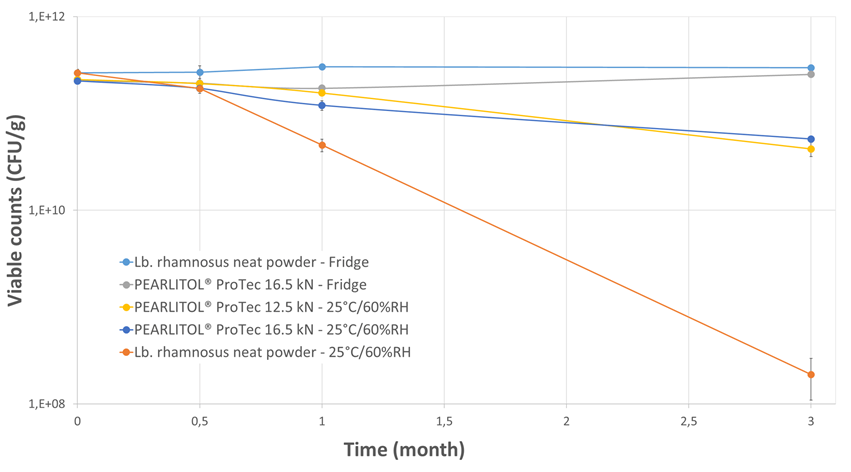
Regardless of the tablet compaction force employed or the storage condition, the tablet hardness (Figure 3), disintegration time (Figure 4), water activity (Figure 5) and water content (Figure 6) were well-maintained throughout the 3-months study duration. The viability of Lb. rhamnosus was also evaluated, and the formulations stored in fridge condition demonstrated stability as expected. However, when the same formulations were stored in the ambient conditions, the decrease in viability for the Lb. rhamnosus neat powder was drastic as compared to those formulated with PEARLITOL® ProTec (Figure 7).
Figure 3. Evolution of tablet hardness during storage for tablets composed of Lb. rhamnosus and PEARLITOL® ProTec. Tablets were produced at two compression forces and stored under two distinct conditions.
Figure 4. Evolution of tablet disintegration time during storage for tablets composed of Lb. rhamnosus and PEARLITOL® ProTec. Tablets were produced at two compression forces and stored under two distinct conditions.
Figure 5. Evolution of tablet water activity during storage for tablets composed of Lb. rhamnosus and PEARLITOL® ProTec. Tablets were produced at two compression forces and stored under two distinct conditions.
Figure 6. Evolution of tablet water content during storage for tablets composed of Lb. rhamnosus and PEARLITOL® ProTec. Tablets were produced at two compression forces and stored under two distinct conditions.
Figure 7. Evolution of probiotic viability during storage under two distinct conditions.
Conclusion
It was demonstrated that the presence of PEARLITOL® ProTec conferred protection to the formulation during compaction and on storage. With storage in ambient conditions, the viability counts could still be maintained at acceptable levels, which highlights the benefit of the low Aw PEARLITOL® ProTec in the formulation.
References
1. Das T.K. et al. Appl Food Res. 2022 Aug:100185.
https://doi.org/10.1016/j.afres.2022.100185
2. Byl E. et al. Eur J Pharm Biopharm. 2019 Dec; 145:7-11.
https://doi.org/10.1016/j.ejpb.2019.10.001
3. Fenster K. et al. Microorganisms. 2019 Mar 17;7(3).
https://doi.org/10.3390/microorganisms7030083
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.