Reverting Reversible Aggregates into Monomers and Arresting Rate of Aggregation of Bevacizumab.
Published March 04, 2026
Extending Shelf Life of Therapeutic Proteins, Part 2
Authors
Rajeev GOKHALE
Head of Global Pharmaceutical Sciences, Roquette America Inc., Geneva, IL 60134, USA
Shiqi HONG
Biopharma Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Tao Peng
BioPharma Research Manager, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Yogesh Kumar Mishra
Senior Research Manager, Biopharmaceutical R&D, Roquette
Situation
The self-association behavior of monoclonal antibodies (mAbs) is concentration-dependent1. Increase in aggregate level is often observed during concentration and buffer exchange steps. Reversible aggregates may subsequently aggregate further to the irreversible aggregated state2. It is hypothesized that hydroxypropyl-ß-cyclodextrin (HPßCD) can interact with protein to interfere with the self-association process, thereby reducing reversible aggregates.
Challenge
In this work, bevacizumab (Avastin®), a monoclonal IgG1 antibody was chosen as the model protein as it is known to aggregate in both reversible and non-reversible aggregate forms. The study aimed to investigate the effect of two HPßCD (KLEPTOSE® HPB, with MS=0.65 and KLEPTOSE® HP, with MS=0.9) on bevacizumab aggregation during (i) buffer exchange and concentration step and (ii) formulation stability.
Solution
Bevacizumab (25 mg/mL) formulated in phosphate buffered saline (PBS) at pH 6.2 was purchased from BOC Sciences (NY, USA). The two biopharma grade HPßCD (KLEPTOSE® HP and KLEPTOSE® HPB) were from Roquette Frères (Lestrem, France).
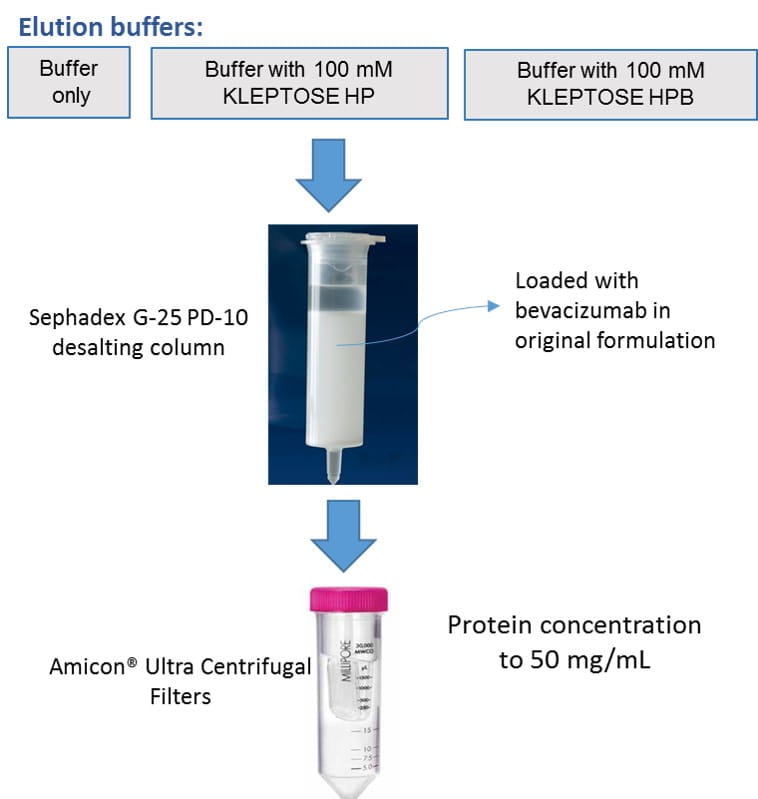
(I) Buffer exchange and concentration steps (Figure 1):
- Bevacizumab in PBS was first diluted 1:1 and then buffer-exchanged into 50 mM sodium phosphate buffer, pH 6.2 in the absence or presence of 100 mM KLEPTOSE® HP or HPB using preparative size-exclusion chromatography column (Sephadex G-25 PD-10).
- An ultrafiltration step using Amicon® ultra centrifugal filters (MWCO= 30KDa) was then performed to concentrate the protein solutions to 50 mg/mL.
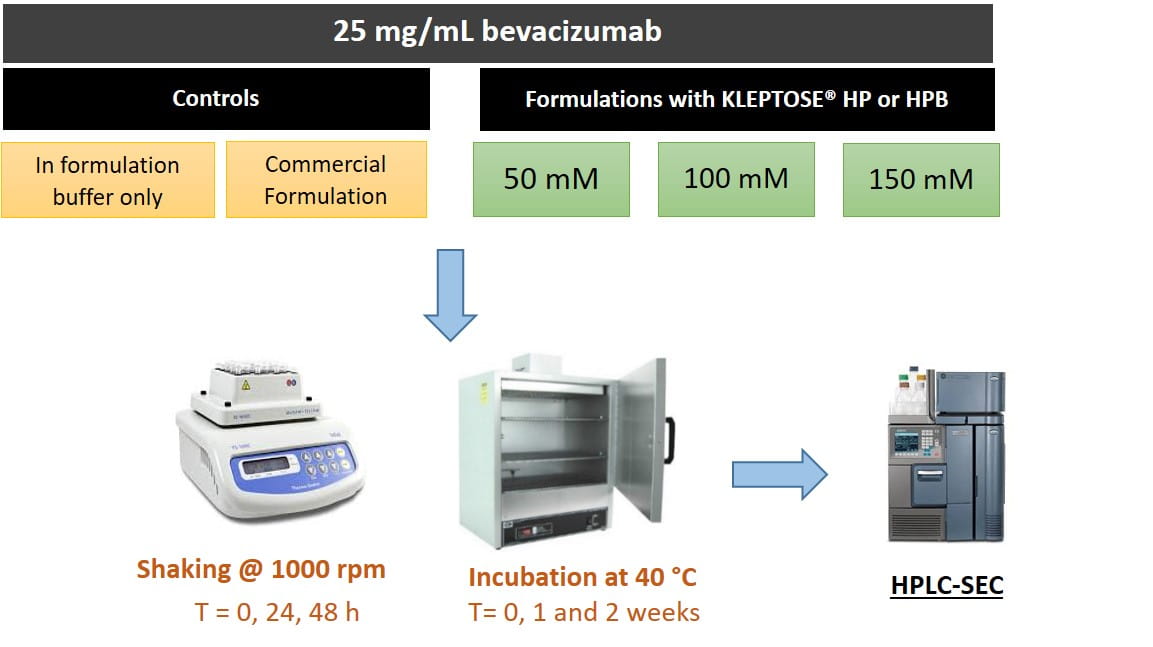
(II) Formulation stability (Figure 2)
- The commercial formulation (CF) of bevacizumab (Avastin®) contains 25 mg/mL bevacizumab in 50 mM sodium phosphate buffer at pH 6.2, 60 mg/mL α,α-trehalose dihydrate and 0.4 mg/mL polysorbate 20.
- In this study, bevacizumab was formulated at 25 mg/mL protein concentration in 50 mM sodium phosphate buffer, pH 6.2 with varying concentration of HPßCD and compared with control formulations: (i) buffer only and (ii) CF.
Results And Discussions
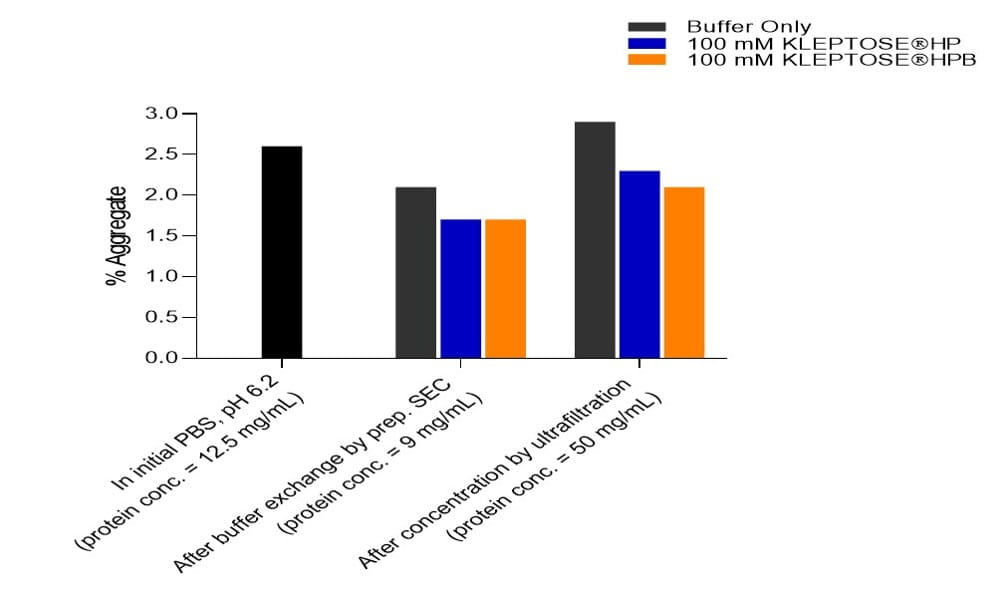
Buffer exchange and concentration steps
In both buffer exchange and ultrafiltration steps, approximately 25% reduction of aggregate was observed in the presence of KLEPTOSE® HP or HPB (Figure 3).
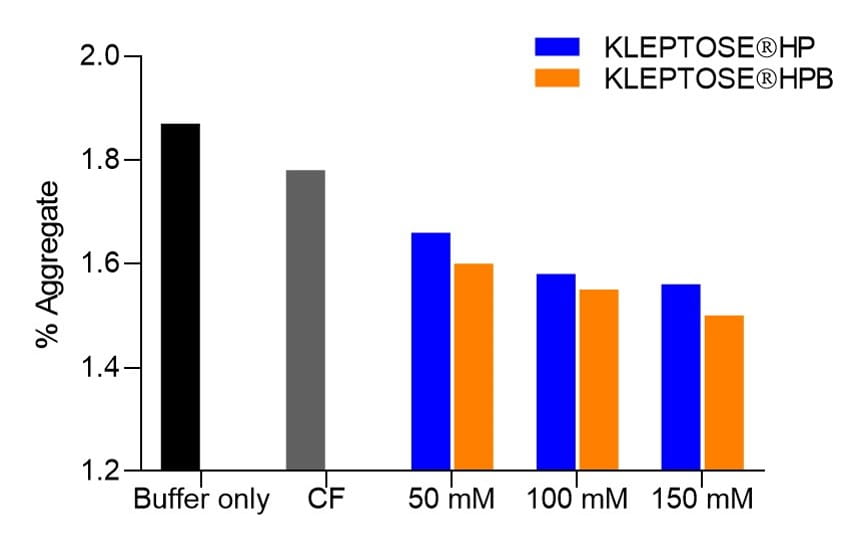
Reduction in reversible aggregates
At T0, addition of KLEPTOSE® HP or HPB in formulations appeared to reduce reversible aggregates in a concentration-dependent manner (Figure 4).
Potential interaction between bevacizumab and KLEPTOSE® HP or KLEPTOSE® HPB may interfere with the self-association of bevacizumab, thereby reducing the formation of reversible aggregates.
Formulation stability
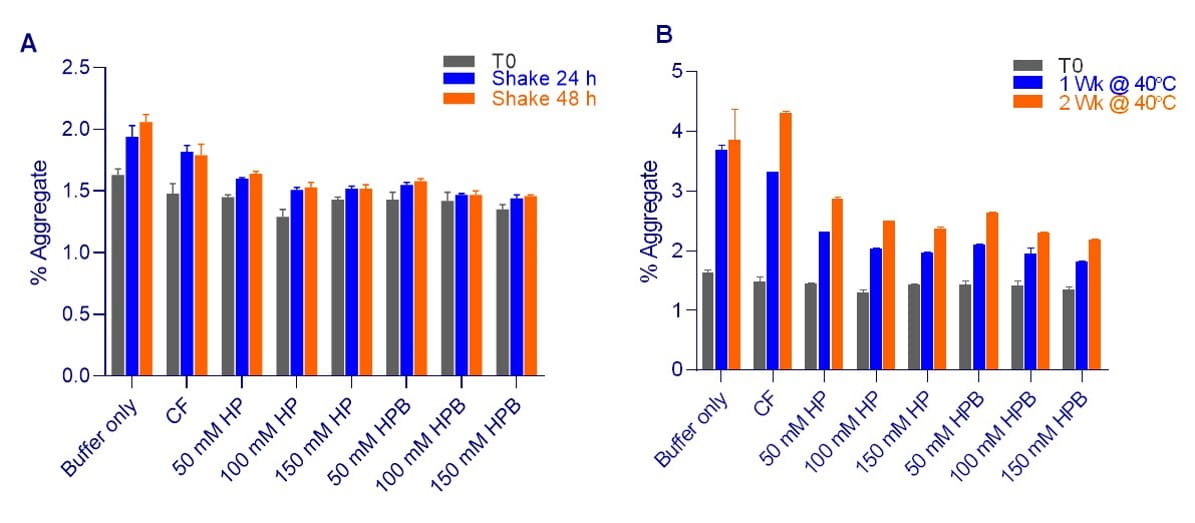
Formulations containing KLEPTOSE® HP or HPB exhibited lower rates of aggregation, as compared to commercial formulation during agitation stress (Figure 5A).
When subjected to thermal stress (Figure 5B), formulations containing KLEPTOSE® HP or HPB exhibited lower rates of aggregation, as compared to commercial formulation (containing 60 mg/mL Trehalose).
Conclusion
- KLEPTOSE® HP and HPB were able to reduce the formation of reversible aggregates in bevacizumab formulations.
- Addition of KLEPTOSE® HP or HPB in buffer was beneficial in reducing aggregates in buffer exchange and concentration steps.
- KLEPTOSE® HP and HPB were also effective in reducing both agitation and thermal stress-induced aggregation, potentially extending the shelf life of bevacizumab formulation.
References
- Shire, S.J., Z. Shahrokh, and J. Liu, Challenges in the development of high protein concentration formulations. Journal of Pharmaceutical Sciences, 2004. 93(6): p. 1390-402.
- Andrews, J.M. and C.J. Roberts, A Lumry-Eyring nucleated polymerization model of protein aggregation kinetics: 1. Aggregation with pre-equilibrated unfolding. The Journal of Physical Chemistry B, 2007. 111(27): p. 7897-913.
Disclaimer
KLEPTOSE® is a registered trademark of Roquette Frères.
Avastin® is a registered trademark of Genentech, Inc.