Arresting Rate of Aggregation During Agitation and Thermal Stress Using IgG as a Model Protein.
Published February 21, 2026
Extending Shelf Life of Therapeutic Proteins - Part 1
Authors
Rajeev GOKHALE
Head of Global Pharmaceutical Sciences, Roquette America Inc., Geneva, IL 60134, USA
Shiqi HONG
Biopharma Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Tao Peng
BioPharma Research Manager, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Yogesh Kumar Mishra
Senior Research Manager, Biopharmaceutical R&D, Roquette
Chiara ZHANG
Analytical Scientist – Pharmaceutical R&D, Roquette
Situation
Protein instability is a major challenge to overcome during manufacturing, formulation, transportation and storage of biopharmaceuticals1. The selection of appropriate excipients to stabilize protein drugs during formulation development is critical in ensuring product stability against various physical stresses throughout its recommended shelf life.
Challenge
In this study, we evaluated the role of hydroxylpropyl ß-cyclodextrin in the stabilization of therapeutic proteins. The stabilizing effects of two hydroxypropyl ß-cyclodextrins (KLEPTOSE® HPB, with MS=0.65 and KLEPTOSE® HP, with MS=0.9) against agitation and thermal stresses on human plasma IgG were investigated. A two-prong approach, using nanoDSF (Differential Scanning Fluorimetry) and size-exclusion chromatography (SEC) HPLC was employed to establish validation.
Solution
Lyophilized human plasma IgG was purchased from BOC Sciences (NY, USA) and reconstituted in purified water at 10 mg/mL. A buffer-exchange step was performed using PD-10 desalting columns (GE Healthcare Life Science, Illinois, USA) to exchange out original formulation into a base buffer of 25 mM sodium phosphate buffer, pH 6.8. The two biopharma grade HPßCD (KLEPTOSE® HP and KLEPTOSE® HPB) were from Roquette Frères (Lestrem, France). All other chemicals were purchased from Merck KGaA (Darmstadt, Germany).
Human plasma IgG was formulated at 5 mg/mL protein concentration in two levels of HPßCD (i.e., 20 mM and 100 mM) and compared with control formulations: (i) buffer only, (ii) 0.05 % w/v Tween ® 80 and (iii) 60 mg/mL trehalose. Formulations were subjected to shaking stress at 1400 rpm using an orbital shaker and thermal stress at 40°C in an oven, respectively. Samples were then analyzed at specific time-points using nanoDSF and SEC.
Results
Surface activity of hydroperoxyde-ß-cyclodextrins
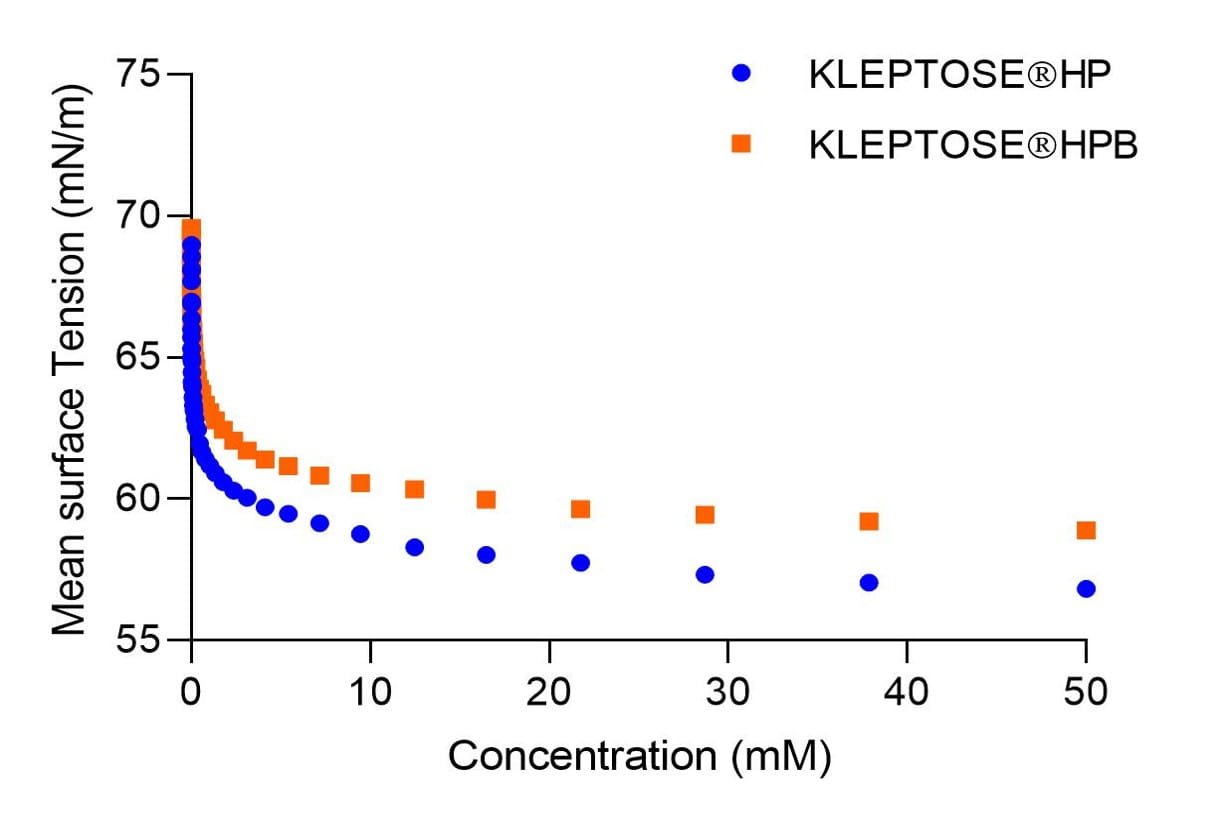
- KLEPTOSE® HP and HPB exhibited surface activity (Figure 1) and can potentially compete with protein at the air-water interface during agitation, inhibiting protein from aggregation.
Figure 1: Surface activity of KLEPTOSE® HP and KLEPTOSE® HPB at various concentrations.
Agitation stress
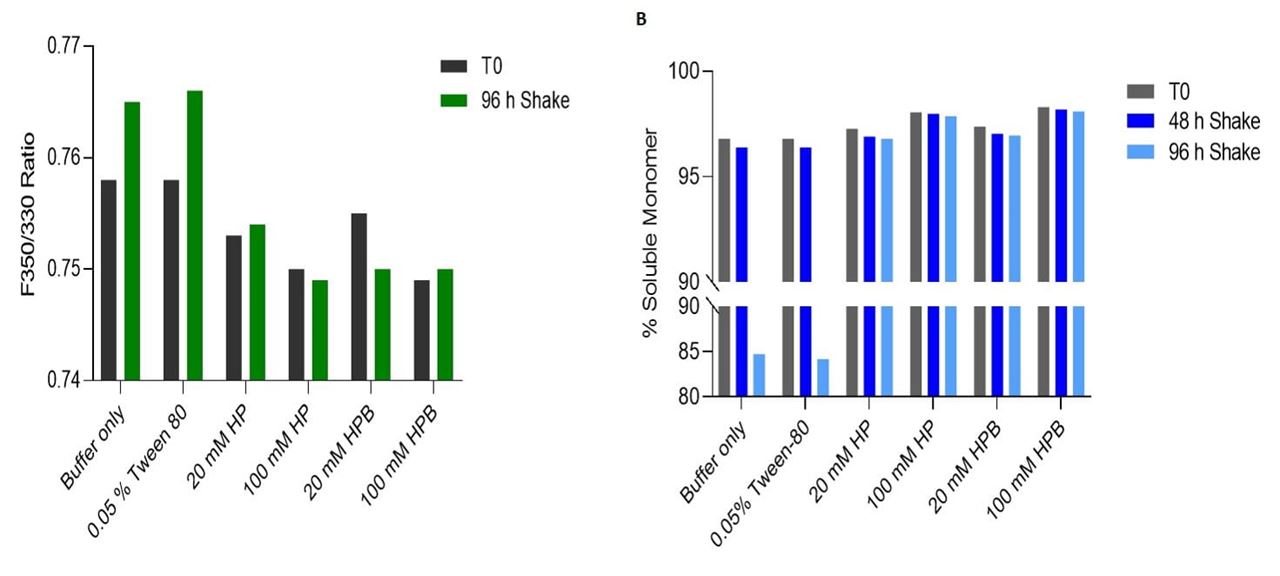
- During agitation stress, lower % unfolding (i.e., lower F350/330 ratio) was observed in formulations containing KLEPTOSE® HP or HPB as compared to controls containing Tween 80 (Figure 2A).
- When analyzed using SEC, formulations containing KLEPTOSE® HP and HPB were able to inhibit fragmentation, resulting in a significantly higher % soluble monomer recovered as compared to controls (Figure 2B).
Figure 2. Stability of IgG after 96h of agitation at 1400 rpm. (A) F350/330 ratio of IgG at T0 and after 96h of shaking. (B) % soluble monomer recovered at T0, after 48h and 96h of shaking.
Thermal stress
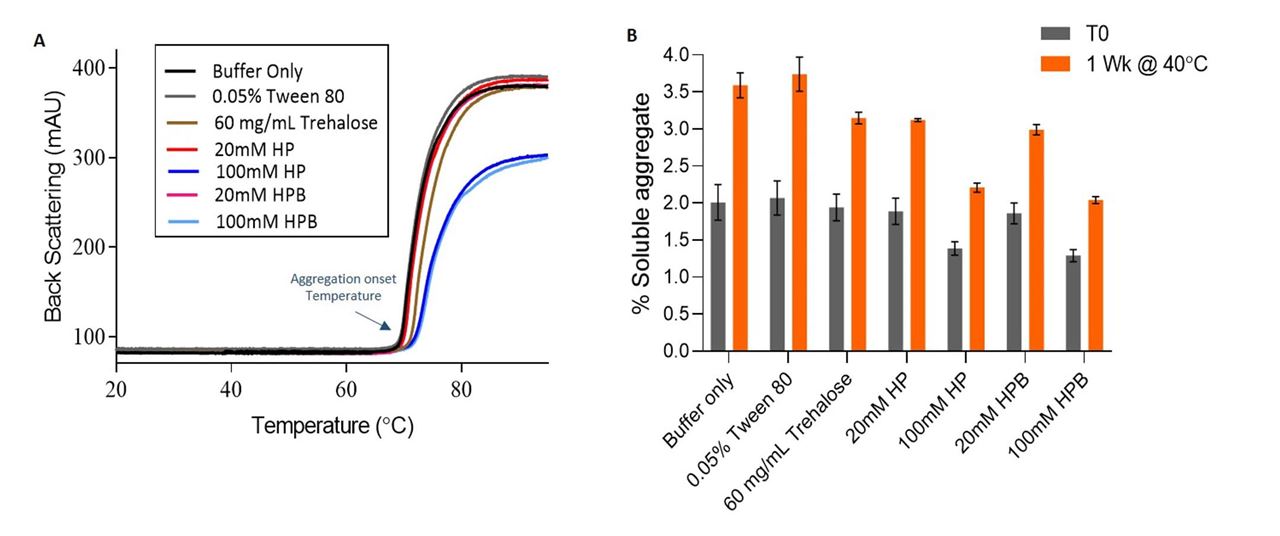
- During thermal ramp, NanoDSF results showed that KLEPTOSE® HP and HPB (100 mM) reduced the relative amount of protein aggregation and increased aggregation onset temperature (Figure 3A).
- Stability study at 40°C also showed that 100 mM KLEPTOSE® HP and HPB were superior in aggregation reduction as compared to trehalose (Figure 3B).
Figure 3. Stability of IgG during thermal stress. (A) Back scattering profile of IgG formulations during thermal ramp from 20 to 95°C (B) % soluble aggregate at T0 and after storage at 40°C for 1 week.
Conclusion
- KLEPTOSE® HP and HPB are surface active and can serve as a functional alternative to surfactants in protein formulations.
- Anti-aggregation properties of KLEPTOSE® HP and HPB confirmed using orthogonal methods (nanoDSF and SEC).
- KLEPTOSE® HP and HPB were effective in reducing both agitation and thermal stress-induced aggregation.
- This dual function stabilization property makes hydroxypropyl ß-cyclodextrin a promising excipient to be explored for therapeutic proteins shelf life extension.
References
- Steinmeyer, D.E. and E.L. Mccormick, The art of antibody process development. Drug Discovery Today, 2008. 13(13-14): p. 613-18.
Disclaimer
KLEPTOSE® is a registered trademark of Roquette Frères.
Tween® is a registered trademark of Croda Americas LLC.