Co-processed Hydroxypropyl Methylcellulose–Mannitol as a Directly Compressible Excipient for Controlled Release
Published February 28, 2026
Presented at the AAPS 2022 PHARMSCI 360, October 16-19, 2022, Boston, MA
Authors
Wen Chin Foo
Pharmaceutical Scientist, Pharmaceutical R&D, Roquette
Keat Theng Chow
Pharmaceutical Research Manager, Pharmaceutical R&D, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Christina Yx Khang
Roquette - National University of Singapore
Kwan Hang Lam
Analytical Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Antoine Salome
Process Engineer – Sugar & Polyol Technologies
Baptiste Bois
Sugars & Polyols Technologies Manager, Roquette
Philippe Lefèvre
Head of Global Pharma Applications Labs, Roquette
Antoine Billa
Global Market Manager, Roquette
Rajeev GOKHALE
Head of Global Pharmaceutical Sciences, Roquette America Inc., Geneva, IL 60134, USA
Paul WS Heng
National University of Singapore
Talles Ernica
Global Technical Developer, Roquette
Introduction
Controlled release dosage forms deliver an effective dose of the active pharmaceutical ingredient over a prolonged duration, which is particularly advantageous to maintain a constant plasma drug concentration within the therapeutic window. This effectively overcomes the negative peak-trough effects associated with multiple dosing of immediate release formulations. In addition, controlled release formulations also feature significantly in patient-centric pharmaceutical design, given the reduced administration frequency hence improved patient compliance. The most common strategy involves dispersing the active ingredient homogeneously in a hydrophilic polymer matrix, which hydrates and swells upon contact with biological fluids to form a viscous diffusion gel barrier which limits drug release. Hydroxypropyl methylcellulose (HPMC) is among the most widely used excipients as a water-soluble, hydrophilic controlled release matrix. However, standard HPMC powders are associated with flow issues due to their fibrous nature, rendering it unsuitable for tableting via direct compression. In this work, PEARLITOL® CR-H HPMC co-processed with mannitol from Roquette Frères was evaluated based on critical quality attributes necessary for a directly compressible, controlled release excipient. In addition to texturization, mannitol also acts to modulate drug release from the polymer matrix.
Materials and Methods
Co-processed HPMC-mannitol was produced by spray-drying whereby HPMC particles were spray coated with mannitol solution. Particle morphology was assessed via scanning electron microscopy of powder samples sputter-coated with platinum. Moisture content was determined by loss on drying using a moisture analyzer (Ohaus® MB 45, USA). The flow properties of co-processed HPMC–mannitol (PEARLITOL® CR-H) were characterized via angle of repose, bulk/tapped density and flow through a 10 mm orifice measurements (Ph. Eur. Chapter 2.9.16), in comparison to direct compression grade HPMC (METHOCEL™ DC2 K4M, Colorcon, USA) and physical mixtures of HPMC–mannitol. The mannitol used in the physical mixture was PEARLITOL® 100 SD from Roquette Frères. Tabletability was evaluated by compacting 400 mg tablets using a compaction simulator (Styl’One Evolution, Medelpharm, France) following Korsch XL-400 simulation with compaction speeds of 63,000 tablets/hour (30 rpm) and 136,500 tablets/hour (65 rpm). A flat-faced, 11.28 mm tooling set, under external lubrication with magnesium stearate was used. Drug release was evaluated with drugs of differing solubilities, namely propranolol hydrochloride (high solubility, 50 mg/mL) and metformin (very high solubility, 300 mg/mL). The controlled release performance of drug-containing co-processed HPMC–mannitol tablets was benchmarked against corresponding commercial products. Tablet shape and drug content were matched with the marketed formulations Ciplar LA 40 mg (Cipla, India) and Glucophage XR 500 mg (Merck). Dissolution studies were conducted with USP dissolution apparatus II, using a physiologically relevant, pH transition method adapted from USP <711> Dissolution Method A.
Results
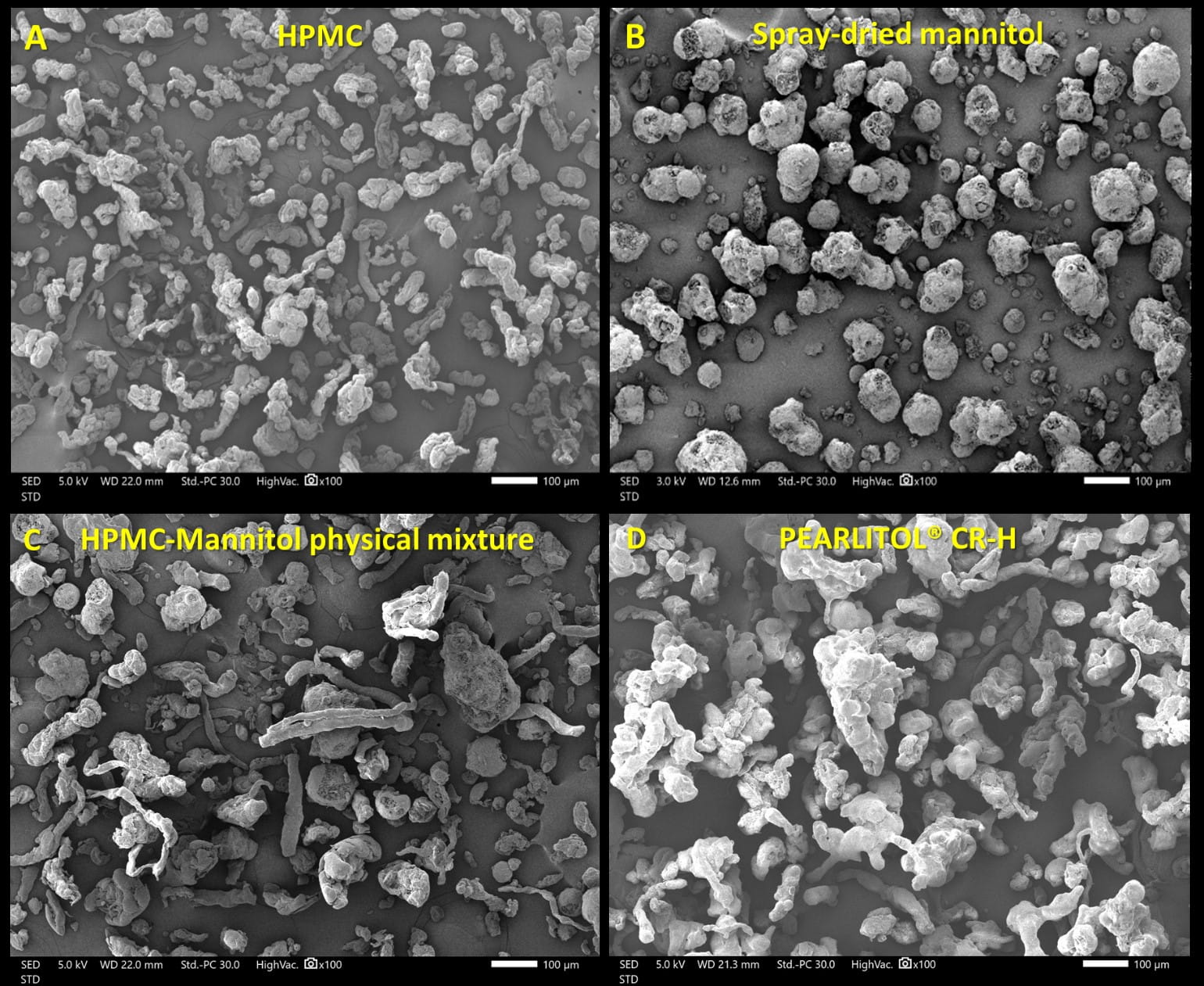
HPMC particles generally appeared elongated and striated when observed under scanning electron microscopy, while spray-dried mannitol, PEARLITOL® 100 SD comprised predominantly spherical, rough particles. Spray coating HPMC particles with mannitol solution resulted in larger, rounder particles for PEARLITOL® CR-H due to deposition of mannitol on the surfaces of HPMC particles (figure 1).
Figure 1. Scanning electron microscopy images of HPMC (A); spray-dried mannitol (B); physical mixture of HPMC and spray-dried mannitol (C); and PEARLITOL® CR-H via fluid bed spray granulation (D). Scale bars represent 100 µm.
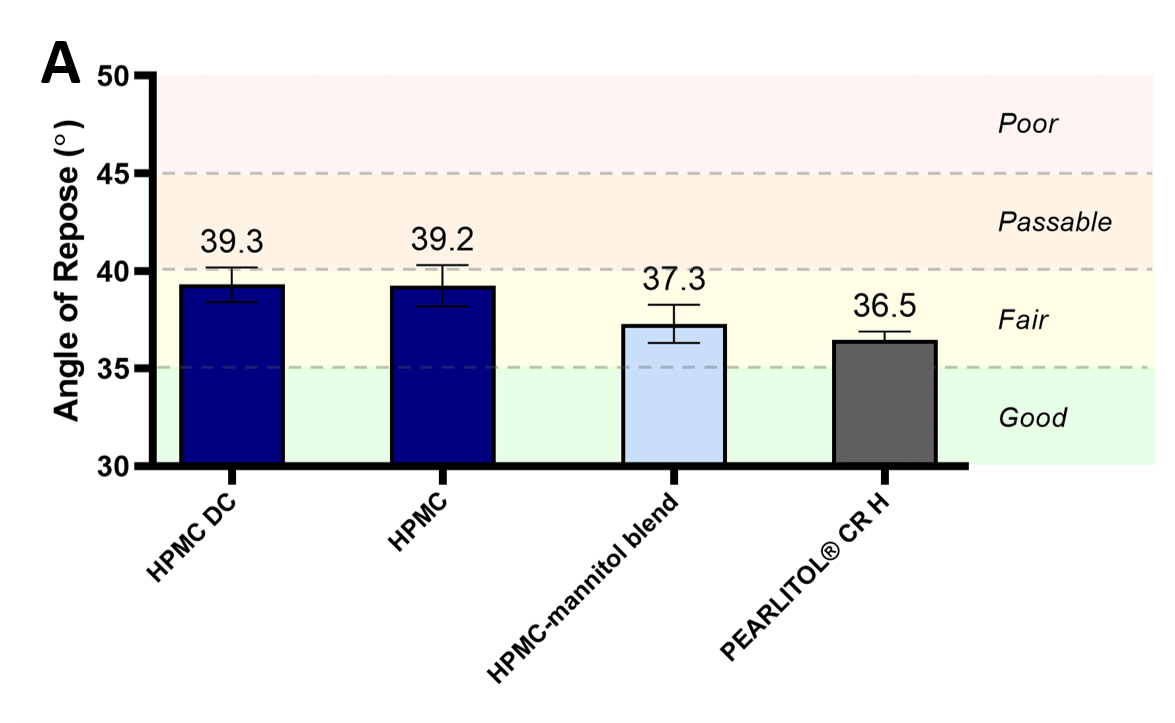
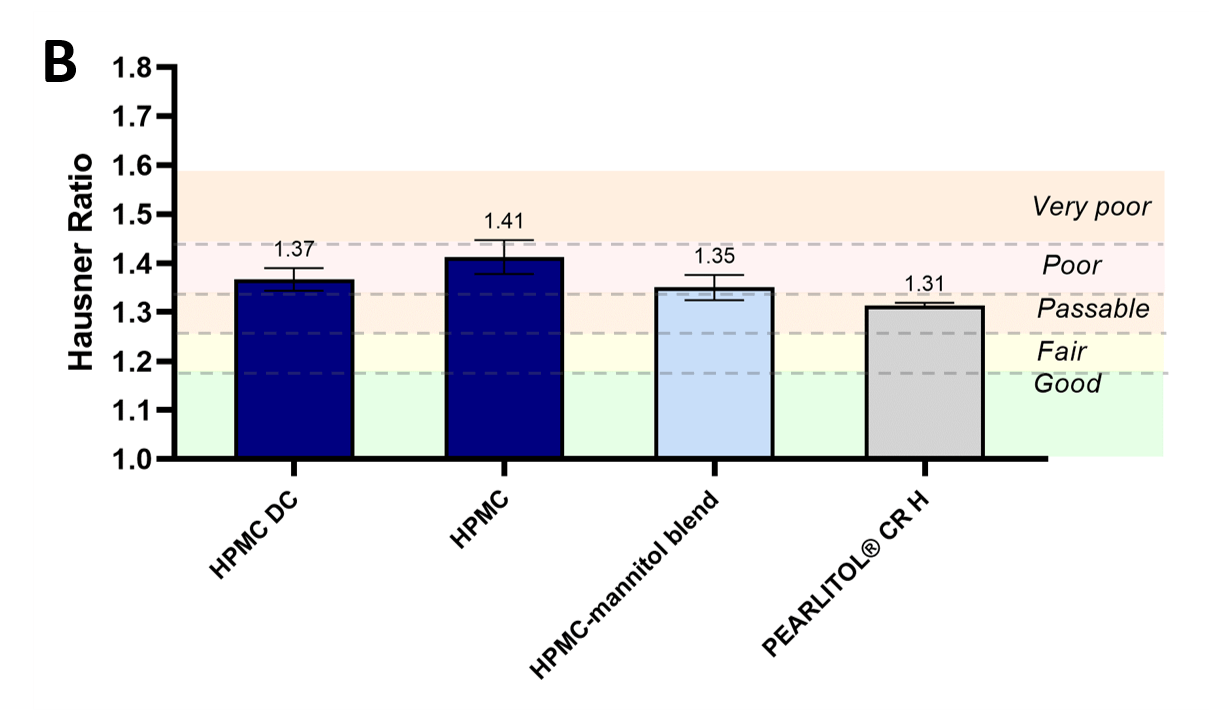
The larger size and rounder shapes of PEARLITOL® CR-H particles was inferred to provide improved powder flowability, as characterized by angle of repose, Hausner ratio and flow time measurements (figures 2[A]) and 2[B], and tables 1 and 2). PEARLITOL® CR-Hshowed excellent flowability based on flow time (12 s/100 g powder). The starting HPMC material recorded an angle of repose of 39.2° (fair flow by USP classification) and Hausner ratio of 1.41 (poor flow by USP classification). Compared to standard HPMC, direct compression HPMC did not demonstrate improvement in angle of repose values, and only slightly better Hausner ratio values showing poor-to-passable flow. The addition of mannitol to HPMC either by blending or co-processing, decreased angle of repose values significantly to 37.3° and 36.6°, respectively. PEARLITOL® CR-H had the smallest Hausner ratio at 1.31 (passable flow).
Figure 2. Flow properties of PEARLITOL® CR-H in comparison to direct compression grade HPMC (HPMC DC), pure HPMC and physical blend of HPMC-mannitol: Angle of repose (A) and Hausner ratio (B).
Table 1. Flow properties of PEARLITOL® CR-H batches using “flow through an orifice” following Ph. Eur. Chapter 2.9.16 – Flowability. Apparatus II was used with a 10mm orifice. 100g of powder was sampled for each measurement.
PEARLITOL® CR-H Batch 01 | PEARLITOL® CR-H Batch 02 | PEARLITOL® CR-H Batch 03 | |
Flow time (seconds/100g) | 12 | 12 | 12 |
Bulk density (g/cm3) | 0.29 | 0.33 | 0.31 |
| Tapped density (g/cm3) | 0.38 | 0.44 | 0.40 |
| Hausner ratio | 1.31 | 1.32 | 1.31 |
| Angle of repose (°) | 36 | 36.7 | 36.6 |
Table 2 presents flow characteristics of powder blends containing a poorly flowable powder (PEARLITOL® 50 C) standing for a poorly flowable active pharmaceutical ingredient (API). Results clearly show a significant improvement of the flowability brought by PEARLITOL® CR-H in comparison to HPMC DC grade and HPMC/mannitol DC grade (PEARLITOL® 100 SD) blend.
Table 2. Flow properties of PEARLITOL® CR-H in comparison to HPMC DC grade and physical blend of HPMC–mannitol using orifice flow method (EP Chapter 2.9.16 – Flowability, Apparatus II, 10mm orifice). 100g of powder was used for each measurement. PEARLITOL® 50C (Roquette Frères, France) was used to model after an API with poor flow. Each formula contains 30% of this poorly flowable powder.
PEARLITOL® CR-H + PEARLITOL® 50 C | HPMC DC grade + PEARLITOL® 50 C | Blend: 70 % HPMC DC grade / 30 % PEARLITOL® 100 SD + PEARLITOL® 50 C | |
| Flow time (seconds/100g) | 8 | No flow | No flow |
| Bulk density (g/cm3) | 0.42 | 0.38 | 0.41 |
| Tapped density (g/cm3) | 0.57 | 0.58 | 0.61 |
| Hausner ratio | 1.37 | 1.53 | 1.49 |
| Angle of repose (°) | 37 | 44 | 39 |
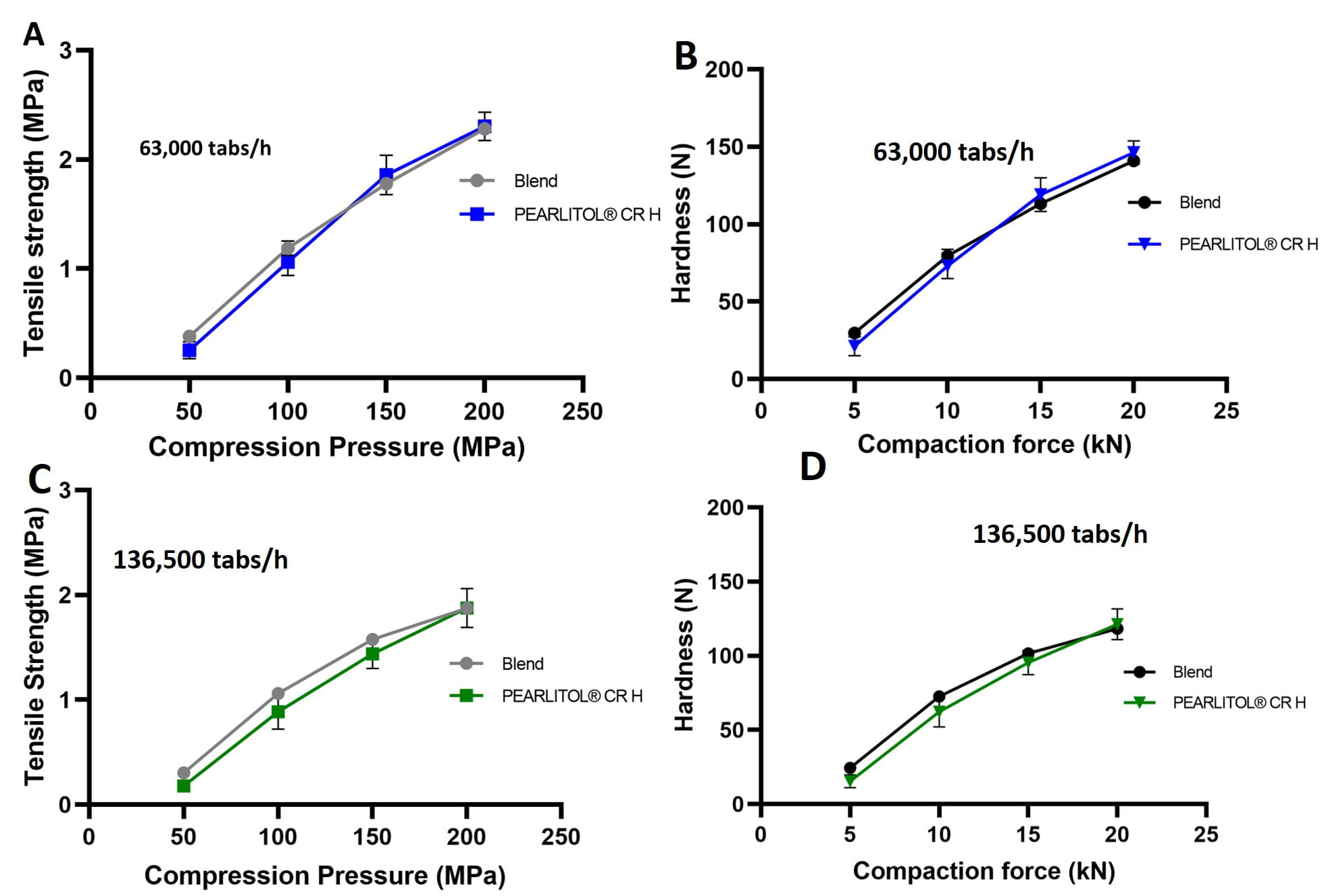
Figure 3 compares the tabletability of PEARLITOL® CR-H and the physical blend HPMC–mannitol at two simulated industrial speeds.
Figure 3. Tabletability of PEARLITOL® CR-H versus physical blend of HPMC–mannitol at two compression speeds of 63,000 tablets/hour – tablet tensile strength (A); hardness (B), and 136,500 tablets/hour – tablet tensile strength (C); hardness (D), following Korsch XL-400 simulation on Styl’One Evolution compaction simulator.
As shown on figure 3, both the physical mixture and co-processed HPMC–mannitol produced tablets of high tensile strength > 1.6 MPa at compression forces of 150–200 MPa which is typical for industrial production.
Figure 4 compares drug release profiles of propranolol HCl and metformin HCl from commercial products and PEARLITOL® CR-H based formula.
Figure 4. Drug release performance of PEARLITOL® CR-H co-processed HPMC–mannitol in comparison to commercial products for metformin HCl (A) and propranolol HCl (B). Similarity factors (f1 and f2) are embedded in the graphs.
The tablets made in-house from PEARLITOL® CR-H exhibit a similar drug release profile when compared to the commercial products for both propranolol (Ciplar 40, Cipla) and metformin (Glucophage XR, Merck), as shown from the similarity factors (figures 4[A] and 4[B]).
Conclusion
PEARLITOL® CR-H is an excellent candidate for a directly compressible, controlled release excipient due to improved flowability and good tabletability. PEARLITOL® CR-H demonstrates excellent controlled release performance for drugs of differing aqueous solubilities.
REFERENCES
1. Boit, B. et al. (2013), United States Patent No. US 2013/0289055 A1.
2. Kang C.Y.X. (2021). A study on site specific drug delivery
METHOCELTM DC2 K4M is a registered trademark of Colorcon, USA.
® Registered trademark(s) of Roquette Frères.
The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without any guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission. For questions about a product’s compliance with additional countries’ standards not listed above, please contact your local Roquette representative.