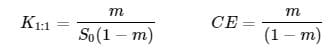Determination of the Thermodynamic Solubility and the Affinity Constants of Carbamazepine, Danazol and Albendazole in KLEPTOSE® HPB Solutions
Published March 07, 2026
Presented at the 26th American Association of Pharmaceutical Scientists annual meeting, 2011, Washington, DC, USA.
Authors
Dr Carmen Popescu
Global Technical Applications Specialist, Roquette
Hassan Almoazen
Department of Pharmaceutical Sciences, University of Tennessee
Wenli Lu
Department of Pharmaceutical Sciences, University of Tennessee
Anthony Samsa
Department of Pharmaceutical Sciences, University of Tennessee
Leon Zhou
Head of R&D - Roquette America
Ashish Joshi
Roquette America
James Johnson
Department of Pharmaceutical Sciences, University of Tennessee
Introduction
The unique cyclic structure of hydroxypropyl beta-cyclodextrin (HPB-CD) enables the formation of host-guest complexes by accommodating a wide variety of drug molecules inside its hydrophobic cavity resulting in the inclusion complexes, where lipophilic compounds are noncovalently bound within the cavity.1,2 The influence of HPB-CD on the solubility of BSC II compounds using phase solubility method (Higuchi and Connors) is a valuable tool to evaluate the possibility of a parenteral alternative to drugs which are commercially available as a solid dosage form.
OBJECTIVES
- To evaluate the phase solubility curve profile, stability constant (K1:1) and the complexation efficiency (CE) of three poorly soluble BCS class II active pharmaceutical ingredients (API) carbamazepine (CBZ), danazol (DZL) and albendazole (ABZ) in hydroxypropyl beta-cyclodextrin (HPB-CD, KLEPTOSE® HPB).
- All three drugs are commercially available as solid dosage forms, but there is no intravenous (IV) alternative.
Materials and Methods
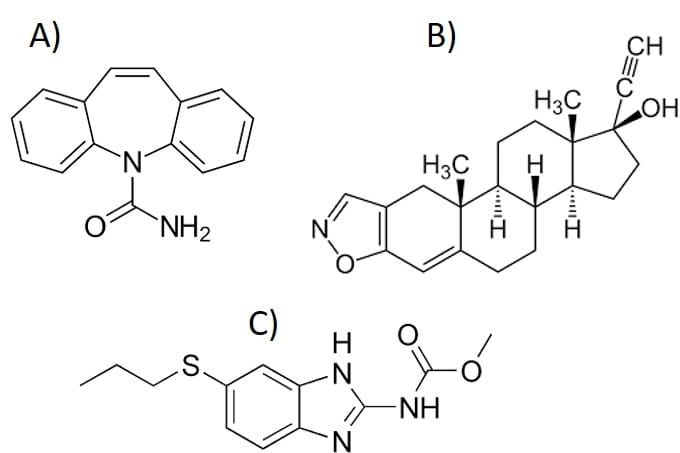
- Relevant chemical information for three drugs tested in this study are shown in table 1.
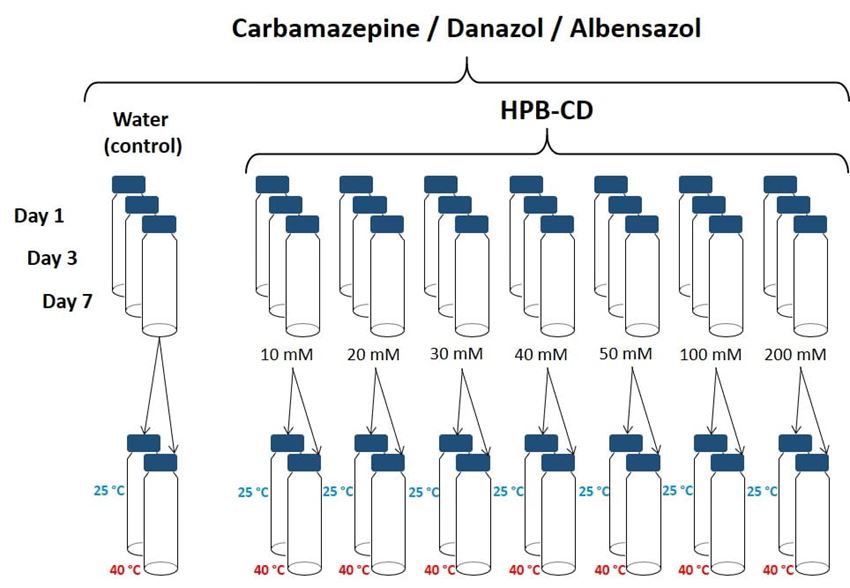
- The phase solubility profile, K1:1 and CE of CBZ, DZL and ABZ were evaluated by adding excess amount of the API to 10, 20, 30, 40, 50, 100 and 200 mM of HPB-CD in deionized water. Samples were evaluated at day 1, 3 (data not shown) and 7 for saturation solubility in order to determine the necessary mixing time at 25 °C. At equilibrium, samples were filtered using Millipore (0.45 μm) syringe filter (as in figure 1). The filtrates were analyzed using the USP analytical HPLC method for CBZ, DZL and ABZ after appropriate dilution.
Figure 1. Phase solubility experiment design
Results And Discussions
Stability Constants (K1:1) and Complexation Efficiency (CE) are calculated as follow:
where m is the slope of the curve of the drug solubility versus HPBCD concentration determined by linear regression and S0 is the drug solubility in DI water as determined after 7 days of mixing.
Figure 2. API molecular structures. (A) Carbamazepine, (B) Danazole, (C) Albendazole.
Table 1. APIs relevant information
| API | Molecular weight | Aqueous solubility | Log P |
| Carbamazepine | 236.26 | <1 mg/l | 2.45 |
| Danazol | 337.46 | <1 mg/l | 4.53 |
| Albendazole | 265.33 | <1 mg/l | 3.22 |
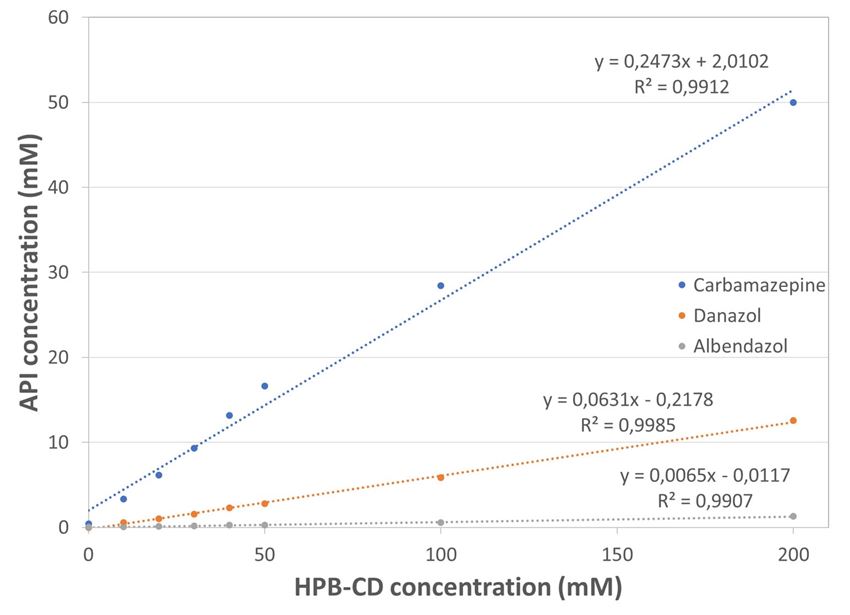
Figure 3. Phase-solubility profiles of carbamazepine, danazol and albendazole in HPB-CD
As per figure 3 (phase solubility diagram), all three APIs are displaying a linear solubility increase as a function of HPB-CD molarity increase indicating a Type AL complexation. Even at low HPB-CD concentration, there is a significant solubility increase for all APIs (table 2) demonstrating that their solubilization by complexation can be a viable solution in formulating them for parenteral delivery.
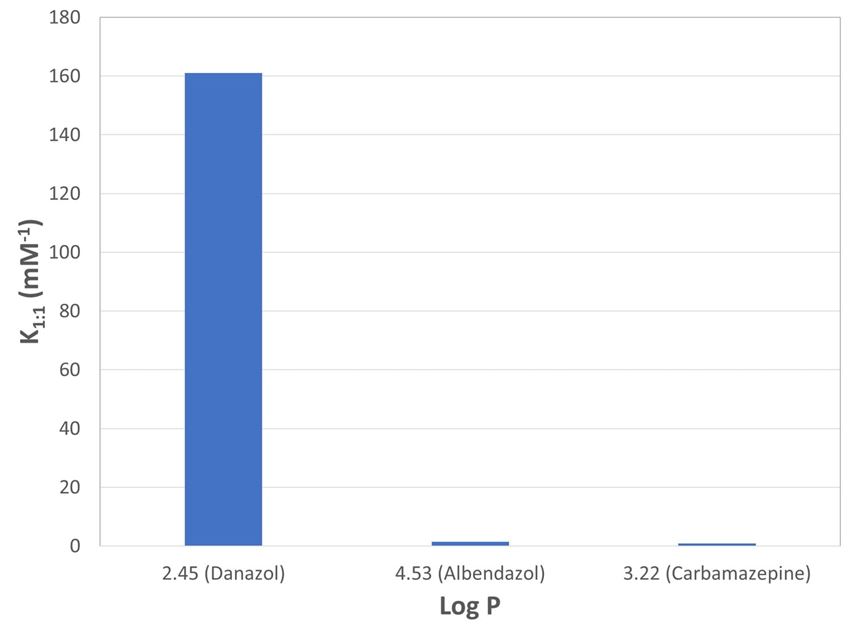
The affinity (stability) constants (K1:1) and complexation efficiencies (CE) of each compound were calculated based on the parameters of the phase solubility graphs. The values are shown in figure 4 and table 3.
Table 2. APIs solubility increase as a function of HPB-CD molarity
HPB-CD | Carbamazepine | Danazol | Albendazole | |||
| Solubility (mg/ml) | S/S0 | Solubility (mg/ml) | S/S0 | Solubility (mg/ml) | S/S0 | |
| 0 | 0.097 | 1 | 0.000142 | 1 | 1.254 | 1 |
| 10 | 0.788 | 8 | 0.193 | 1359 | 20.181 | 16 |
| 20 | 1.45 | 15 | 0.34 | 2394 | 37.178 | 30 |
| 30 | 2.197 | 23 | 0.523 | 3683 | 46.806 | 37 |
| 40 | 3.107 | 32 | 0.774 | 5451 | 70.376 | 56 |
| 50 | 3.927 | 40 | 0.94 | 6620 | 74.153 | 59 |
| 100 | 6.723 | 69 | 1.983 | 13965 | 146.353 | 117 |
| 200 | 11.805 | 122 | 4.239 | 29852 | 352.701 | 281 |
Figure 4. The affinity (binding) constants of each compound
Table 3. API-cyclodextrin affinity constants and complexation efficiencies
| API | S0 (mM) | m | K1:1 (mM-1) | CE | Log P |
| Carbamazepine | 0.411 | 0.2445 | 0.79 | 32.36 % | 2.45 |
| Danazol | 0.000421 | 0.0636 | 161 | 6.79 % | 4.53 |
| Albendazole | 0.00473 | 0.0065 | 1.38 | 0.65 % | 3.22 |
Conclusion
- When comparing Log P of these APIs with their K1:1 (table 3 and figure 4), it was noted danazol with the highest Log P (4.53) value has the highest affinity constant value (161) while carbamazepine has the lowest one K1:1 = 0.79 (also lowest Log P = 2.45). Considering the steric structure of these three molecules (see table 1), carbamazepine with a tricycles structure has the highest complexation efficiency (32.36 %). Based on these findings, one can hypothesize that the CBZ has more binding sites with HPB-CD compared to the other two molecules.
- Danazol has the highest solubility time increase even at low HPB-CD molarity.
- All three drugs’ (CBZ, DZL, ABZ) solubility is significantly increased by HPB-CD complexation making them good candidates for IV formulation.
References
1. Loftsson T, Jarho P, Másson M, Järvinen T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005 Mar;2(2):335-51.
https://doi.org/10.1517/17425247.2.1.335
2. Loftsson T, Másson M, Brewster ME. Self-association of cyclodextrins and cyclodextrin complexes. J Pharm. Sci. 2004 May;93(5):1091-9
https://doi.org/10.1002/jps.20047
® Registered trademark(s) of Roquette Frères.
The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without any guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission. For questions about a product’s compliance with additional countries’ standards not listed above, please contact your local Roquette representative.