SODIUM GLUCONATE CRYSTAL PHARMA
- Physiological compatible electrolyte as API
- Buffering agent as Excipeint
Sodium organic salt,
Applications
- Injectable
- Large Volume Parenterals (LVP)
- Rx Injectable
Functional properties
- APIs
- Physiological electrolyte agent
- Excipients
- Buffer agent
Documents
Product Specification Sheet
Name
Region
Size
Download
SODIUM GLUCONATE PHARMA
465,99 Ko
Region :
465,99 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Compliance | USP-NF |
|---|---|
| CAS number | 527-07-1 |
| Physical form or apperance | Yellowish crystalline powder |
| Application | Sodium Gluconate Pharma is a pyrogen-free substance that is used for parenteral preparations as well as in Rx injectables. As an API it serves as a physiological electrolyte agent in large volume parenteral applications. As an excipient it is used as a buffer agent in Rx injectables. |
| Teste/Odor | Odorless |
| Morphology |
|
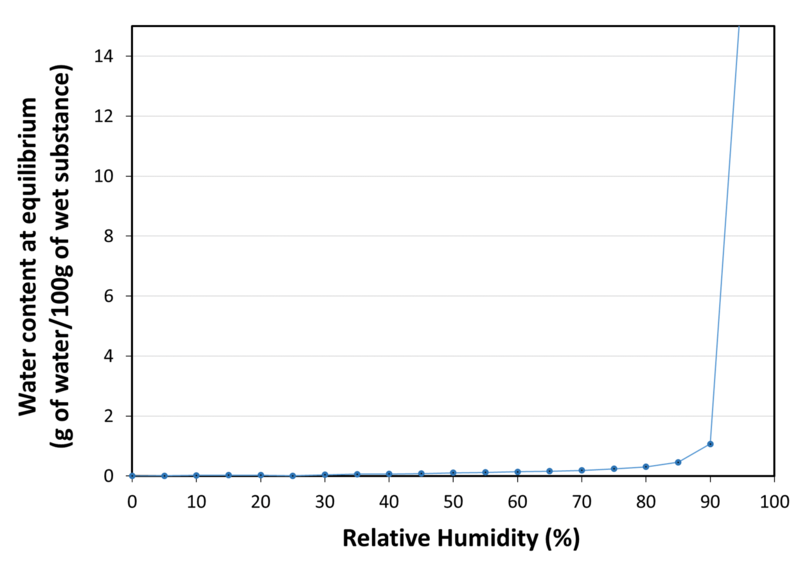
| Water sorption isotherm at 20°C |
|
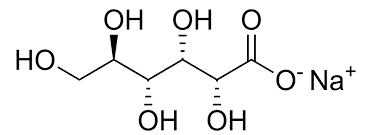
| Chemical Structure |
|
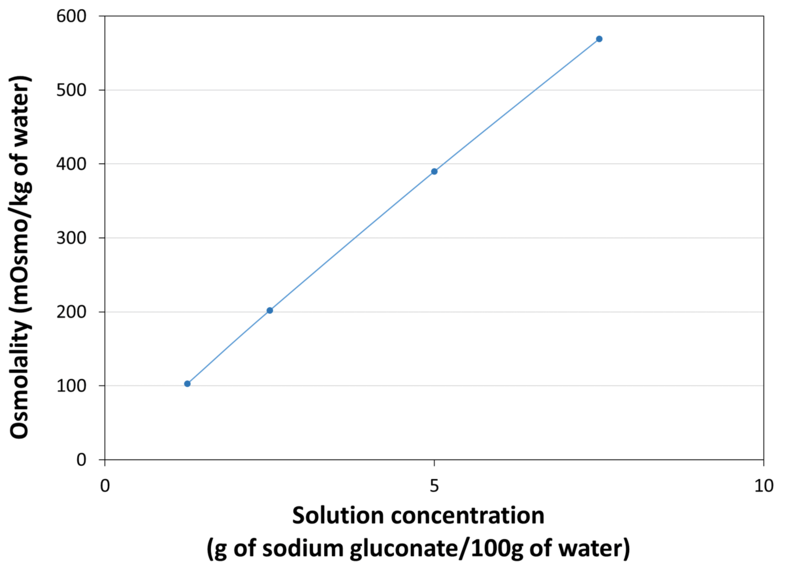
| Osmolality |
|
| Average molecular weight | 218.14 g/mol |
| Solubility | Freely soluble in water (59 g/100 ml of water at 25 °C) |
| Average mean particle diameter | 115 |
| dv10 Particle size distribution | 67 |
| dv50 Particle size distribution | 113 |
| dv90 Particle size distribution | 121 |