PEARLITOL® 200 GT - MANNITOL
PEARLITOL®
®Registered Trademark(s) of Roquette Frères
- Direct Compression
- Reduce capping risk
- Improve tabletting speed
Narrow particle size
Applications
- 1. Solid Forms - Tablets
- Chewable Tablets
- Swallowable Tablets
- Orally Dispersible Tablets
- Effervescent Tablets
- 2. Solid Forms - Capsules
- Hard Capsules Fill
- 5. Other Solid Forms
- Granules and Pellets
- 3. Films and coatings
- Film Coating
Functional properties
- Formulations Aids
- Fillers and Binders for Direct Compression
- Fillers and Binders for Roller Compaction
- Sensory Enhancers
- Sweetening agent
- Stabilizers and Adjusters
- Stabilizers
Documents
Product Specification Sheet
Name
Region
Size
Download
PEARLITOL® 200 GT-EXP
482,14 Ko
Region :
482,14 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
PEARLITOL® 200 GT-EXP
Oceania, AU
EN
272,87 Ko
Region : Oceania, AU
EN
272,87 Ko
PEARLITOL® 200 GT-EXP
Europe, BE
EN
518,74 Ko
Region : Europe, BE
EN
518,74 Ko
PEARLITOL® 200 GT-EXP
Europe, CY
EN
515,90 Ko
Region : Europe, CY
EN
515,90 Ko
PEARLITOL® 200 GT-EXP
Europe, DE
EN
521,88 Ko
Region : Europe, DE
EN
521,88 Ko
PEARLITOL® 200 GT-EXP
Americas, CA
EN
287,68 Ko
Region : Americas, CA
EN
287,68 Ko
PEARLITOL® 200 GT-EXP
Europe, CH
EN
518,98 Ko
Region : Europe, CH
EN
518,98 Ko
PEARLITOL® 200 GT-EXP
Asia, CN
EN
328,31 Ko
Region : Asia, CN
EN
328,31 Ko
PEARLITOL® 200 GT-EXP
Europe, AT
DE
528,62 Ko
Region : Europe, AT
DE
528,62 Ko
PEARLITOL® 200 GT-EXP
Europe, BE
DE
531,58 Ko
Region : Europe, BE
DE
531,58 Ko
PEARLITOL® 200 GT-EXP
Europe, BE
FR
632,29 Ko
Region : Europe, BE
FR
632,29 Ko
PEARLITOL® 200 GT-EXP
Europe, BE
NL
527,00 Ko
Region : Europe, BE
NL
527,00 Ko
PEARLITOL® 200 GT-EXP
Americas, BR
PT
538,35 Ko
Region : Americas, BR
PT
538,35 Ko
PEARLITOL® 200 GT-EXP
Europe, BG
BG
676,98 Ko
Region : Europe, BG
BG
676,98 Ko
PEARLITOL® 200 GT-EXP
Europe, CY
EL
669,33 Ko
Region : Europe, CY
EL
669,33 Ko
PEARLITOL® 200 GT-EXP
Europe, CY
TR
669,86 Ko
Region : Europe, CY
TR
669,86 Ko
PEARLITOL® 200 GT-EXP
Europe, DE
DE
534,56 Ko
Region : Europe, DE
DE
534,56 Ko
PEARLITOL® 200 GT-EXP
Americas, CA
FR
401,42 Ko
Region : Americas, CA
FR
401,42 Ko
PEARLITOL® 200 GT-EXP
Europe, CH
DE
531,84 Ko
Region : Europe, CH
DE
531,84 Ko
PEARLITOL® 200 GT-EXP
Europe, CH
FR
632,84 Ko
Region : Europe, CH
FR
632,84 Ko
PEARLITOL® 200 GT-EXP
Europe, CH
IT
620,61 Ko
Region : Europe, CH
IT
620,61 Ko
PEARLITOL® 200 GT-EXP
Asia, CN
ZH
558,73 Ko
Region : Asia, CN
ZH
558,73 Ko
PEARLITOL® 200 GT-EXP
Europe, CZ
CS
649,78 Ko
Region : Europe, CZ
CS
649,78 Ko
PEARLITOL® 200 GT-EXP
Europe, EE
ET
622,74 Ko
Region : Europe, EE
ET
622,74 Ko
PEARLITOL® 200 GT-EXP
Europe, DK
DA
527,47 Ko
Region : Europe, DK
DA
527,47 Ko
PEARLITOL® 200 GT-EXP
Europe, ES
ES
533,81 Ko
Region : Europe, ES
ES
533,81 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
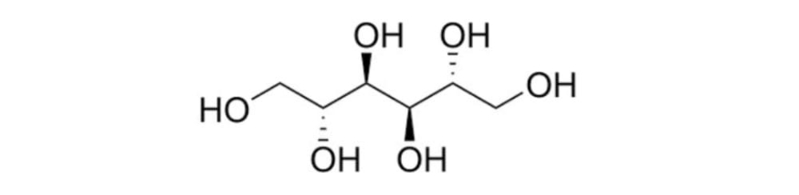
| Synonyms | D-Mannitol |
|---|---|
| CAS number | 69-65-8 |
| Physical form or apperance | White or almost white crystalline powder |
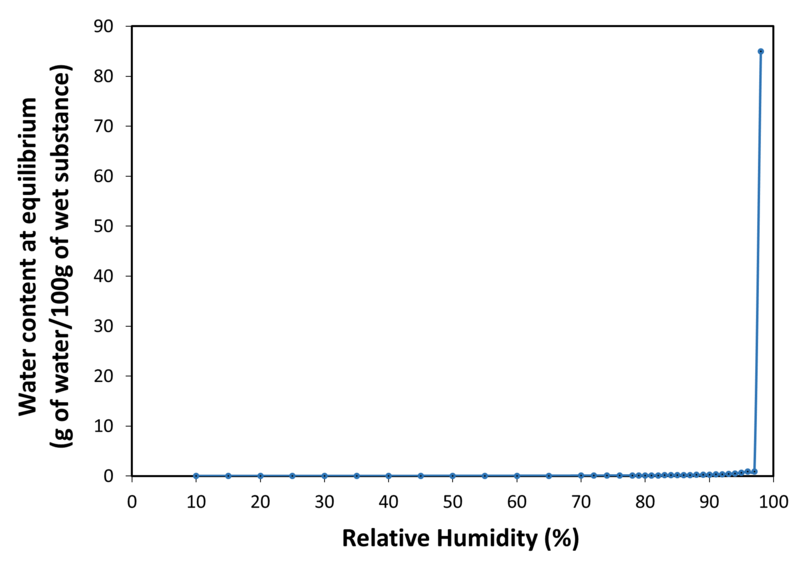
| Application | PEARLITOL® 200 GT mannitol is a direct compression excipient, specially designed to avoid capping, improve flowability and tabletability. It is a granulated form of mannitol with exceptional physical and chemical stability. It offers high API compatibility and no hygroscopicity. It is also a non-cariogenic and non-acidogenic sugar-free sweetener. It is suitable in formulations addressing all types of patient populations including pediatric and diabetic. It can be used in tablets (lozenges, swallowable tablets, orally dispersible tablets, chewable tablets, and effervescent tablets) and in powder blends (sachets, oral stick pack and hard gelatin capsules filling). |
| Teste/Odor | Slightly sweet and cooling effect. |
| Morphology |
|
| Water sorption isotherm at 20°C |
|
| Chemical Structure |
|
| Average molecular weight | 182.2 g/mol |
| Maximal Water content (LOD) | 0.50 |
| Solubility | Freely soluble in water (1 part in 5.5 part of water at 20°C), sparingly soluble in 95% ethanol (1 part in 83), practically insoluble in ether |
| Minimum melting temperature | 165 °C |
| Maximum melting temperature | 170 °C |
| Average mean particle diameter | 160 |
| dv10 Particle size distribution | 90 |
| dv50 Particle size distribution | 150 |
| dv90 Particle size distribution | 260 |
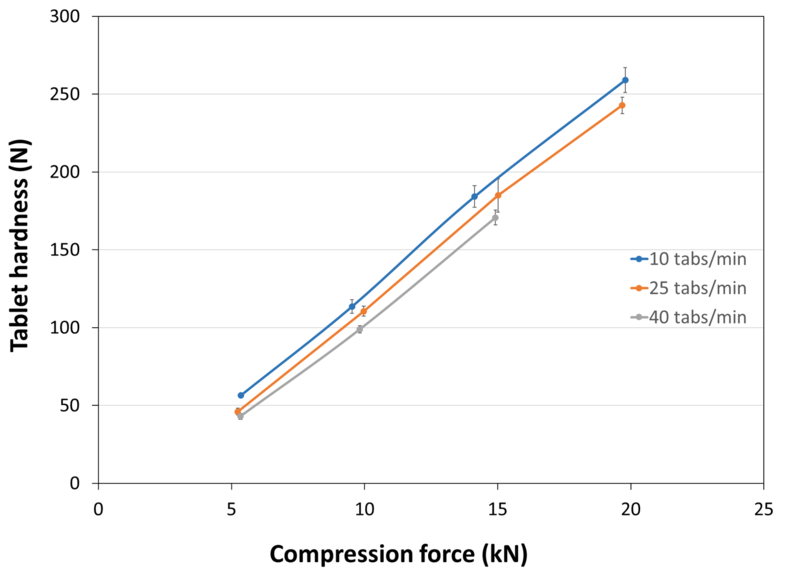
| Tablet Hardness |
|
| Experimental Conditions for Compression Behavior_Tablet Press | STYL'ONE EVO |
| Experimental Conditions for Compression Behavior_Production Speed | 10, 25 and 40 tablets/min (respective linear punch velocity: 38, 96 and 152 mm/s ; respective simulated rotary press speed: 60000, 150000 and 240000 tablets/hour) |
| Experimental Conditions for Compression Behavior_Tooling | Diameter 10 mm R9 concave |
| Experimental Conditions for Compression Behavior_Formuls | 98.8% PEARLITOL® 200 GT / 1.2% magnesium stearate |
| Experimental Conditions for Compression Behavior_Tablet Mass | 400 mg |
| Powder Flowability (according to Ph.Eur. 2.9.16, 10mm outflow opening) | 4 |
| Bulk Density (g/ml) | 0.63 |
| Tapped Density (g/ml) | 0.75 |
| True Density (g/ml) | 1.51 |
| Specific Surface Area (m²/g) | 1.30 |
| Angle of Repose (°) | 27 |