New Research Reveals a Promising Functional Alternative to Surfactants within Biologic Formulations
Published February 21, 2026
Author :
Ferguson Peter
Pharma Global Market Manager
Tao Peng
BioPharma Research Manager, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Overview
One of the most common challenges when formulating biological molecules for therapeutic use is overcoming the molecules’ tendency to aggregate. Currently, the nonionic surfactants polysorbate 80 and polysorbate 20 are widely used to stabilize proteins in liquid preparations for parenteral administration, but there are concerns about both their composition and stability. In small-molecule formulations, cyclodextrins and their derivatives are well established, enabling excipients, and are already approved for parenteral use. New research points to these excipients as a promising alternative to surfactants for biologics.
Protein Formulation Issues
The inherent instability of proteins, especially those that are genetically engineered and far removed from their native environment, makes them challenging to work with. Proteins are sensitive to temperature changes, shearing, shaking, solvents, ionic strength, purity, protein concentration, pressure, and freeze/thaw cycles; they only remain stable within a narrow pH range and are susceptible to adsorption. Of the various degradation pathways that occur, aggregation is one of the most common and is a significant cause for concern since protein aggregates reduce drug efficacy and can induce immunogenicity. Effective formulation plays a critical role in successfully producing a stable protein drug.
The Excipient Challenge
A biological formulation contains multiple excipients, each one contributing to a different desired outcome. A wide range of excipient materials is approved for parenteral applications, and in liquid formulations, polysorbates are often used to protect against interface-induced protein aggregation and surface adsorption. Some 70% of marketed formulations of monoclonal antibody products contain either polysorbate 20 or polysorbate 80.
However, there are two main concerns when formulating a protein drug product using polysorbate. Firstly, the excipient itself is not a discrete molecule, and secondly, there are worries about excipient stability. Polysorbates 20 and 80 are chemically diverse mixtures that contain mainly sorbitan polyoxyethylene esters of fatty acids. These esters are prone to degradation by autoxidation and hydrolysis with the generation of peroxides that can promote protein instability. Therefore, degradation of polysorbates raises questions about both the lowered ability of the surfactant to protect the formulation against interfacial stresses and the impact of the degradation products on the stability of the protein.
Exploring Cyclodextrins
Cyclodextrins and their derivatives are widely employed as excipients within small-molecule applications in which they are used to enhance solubility and bioavailability, improve drug chemical and physical stability, and deliver taste-masking. When formulating for parenteral delivery, it is necessary to use a modified cyclodextrin in order to achieve the required water solubility, and hydroxypropyl-β-cyclodextrin (HPβCD) is well established here. Its unique structure, which includes a hydrophilic exterior and a hydrophobic cavity, enables the formation of inclusion complexes in which lipophilic compounds such as Active Pharmaceutical Ingredients (APIs) are non-covalently bound within the cavity. This increases the API solubility and may improve the taste or create a higher bioavailability for the drug. Extensive toxicological and pharmacological studies have shown that HPßCD is safe for parenteral application and it is currently used in approved products such as itraconazol (Sporanox®) and Mitomycin (MitoExtra®). However, all of these are exclusively low-molecular weight drugs.
Previous work on HPßCD in protein formulations suggests a mechanism of action in which the hydrophobic interior of the HPßCD cavity complexes to the exposed hydrophobic amino acid residues on the protein [1]. Since the exterior of HPßCD is hydrophilic, this effectively shields hydrophobic interactions and blocks the protein-protein interactions that lead to aggregation. It is also thought that HPßCD can inhibit protein aggregation induced by exposure to the air-water interface by acting in a manner similar to nonionic surfactants and displacing proteins from that interface.
Assessing HPßCD for Protein Applications
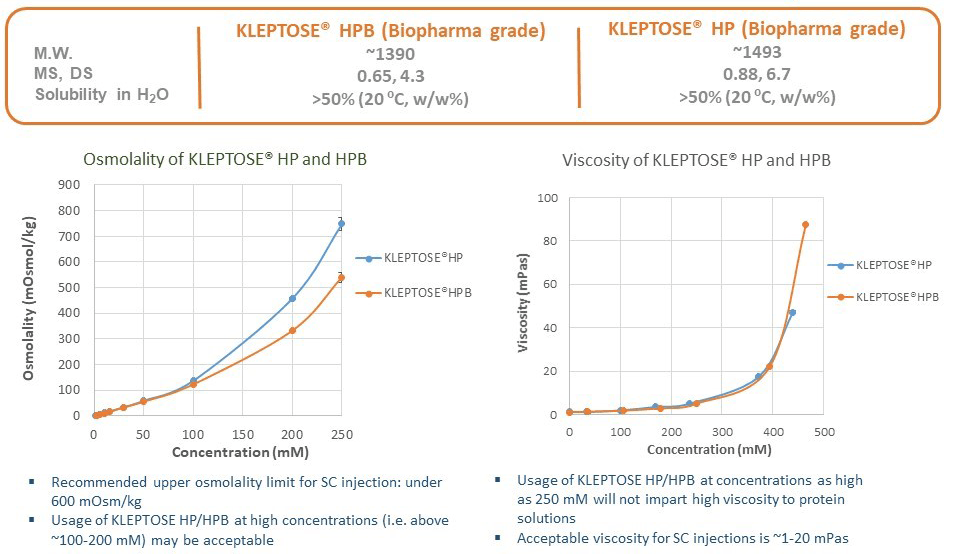
Scientists at Roquette have carried out experimental work to further explore the properties of HPßCD and examine its utility for real-world protein formulations. Two biopharmaceutical grade products were used throughout the studies—Roquette’s KLEPTOSE® HP and KLEPTOSE® HPB. Both products exhibit surface-active properties in addition to other key physical properties (Figure 1).
In preliminary studies, differential scanning fluorimetry (NanoDSF, Prometheus) was used to understand the effects of these HPßCD products on the thermal stability and thermal unfolding of human growth hormone and the monoclonal antibody infliximab. Each was formulated with a range of different HPßCD concentrations and subjected to NanoDSF analysis. For both proteins, increasing the HPßCD concentration in the formulation resulted in higher melting temperatures and a decrease in the backscattering of light that results from the presence of protein aggregates. Overall, the total aggregate level decreased in the presence of 100 mM quantities of both of the HPßCD products tested, indicating enhanced thermal stability. The following case studies report additional work to assess the effects of these two HPßCD products on proteins under stressed conditions, including agitation and thermal stress at 40°C.
Case Study 1: Arresting Rate of Aggregation during Agitation and Thermal Stress Using IgG as a Model Protein
In this experiment, human-plasma high-purity Immunoglobulin (IgG) was reconstituted into 25 mM phosphate buffer at pH 6.8 before formulation with different excipients. The sample set consisted of controls and test materials. Controls were IgG in buffer (i) with no additives, (ii) with the addition of 0.05% polysorbate 80, and (iii) with 60 mg/ml trehalose. Tests were IgG in buffer formulated with HPßCD (either Kleptose® HP or HPB) at 20 and 100 mM. The concentration of IgG in all tubes was 5 mg/ml.
Sample sets were subjected to agitation and heat stress. Subsequent analysis employed NanoDSF to examine thermal stability and size exclusion chromatography–high-performance liquid chromatography (SEC–HPLC) to determine protein monomer, aggregate, and fragment content. For the agitation study, samples were shaken on an orbital shaker (Eppendorf Thermomixer® C) at 1400 rpm at room temperature and were analyzed after 10, 24, 48, and 96 hours of shaking. For the heat stress study, samples were maintained at 40]C for one week prior to analysis.
NanoDSF was carried out on all samples together using a temperature ramp of 20–95 degrees with an increase of 1.5 degrees per minute. The unfolding profile, indicated by fluorescence at 350/330 nm, and the relative amounts of protein aggregation, indicated by the degree of backscattering, were obtained simultaneously. SEC–HPLC (Waters Acquity Arc HPLC/PDA detector/Empower 3) was run at a wavelength of 218 nm and the area under the curve for each peak was calculated to determine the percentage of aggregate, monomers, and fragments.
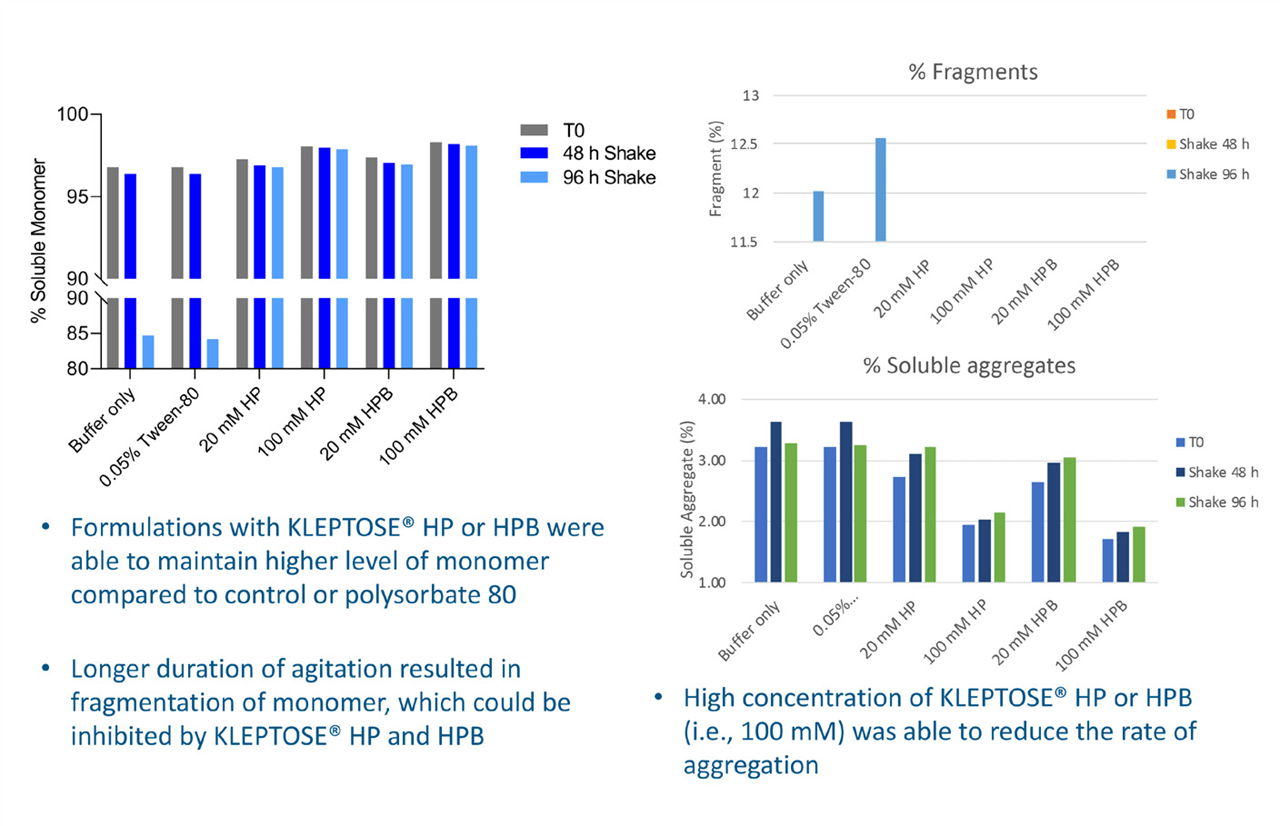
Effects of agitation
NanoDSF comparison of the fluorescence levels at 350/330 before and after shaking indicated that after 96 hours, the proteins in samples containing HPßCD underwent less unfolding than in the buffer alone or in buffer with polysorbate. Evaluation by SEC–HPLC showed that these samples also maintained a higher level of monomer with a negligible decrease in monomer percentage (see Figure 2). The extensive monomer fragmentation observed after 96 hours in the controls was not replicated where HPßCD was present. High concentrations (100 mM) of both the commercial HPßCD products also reduced aggregation rates.
Effects of thermal stress
During thermal ramping of NanoDSF, the level of backscattering of light allows comparison of relative aggregation levels. NanoDSF analysis showed that samples containing 100 mM concentrations of either of the HPßCD excipients exhibited less backscattering than the other samples, with a significant approximately 25% reduction in aggregation and a higher temperature of onset.
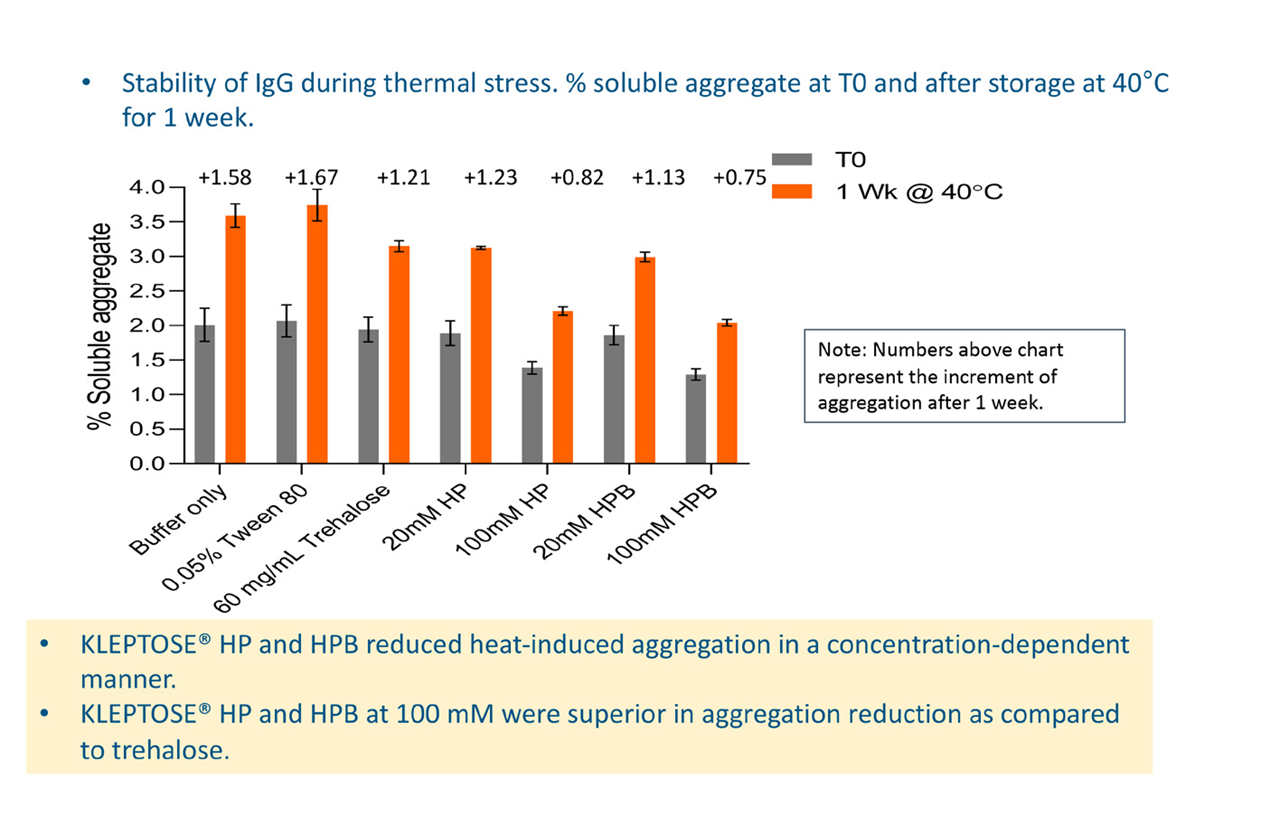
SEC–HPLC analysis showed that after one week of heat stress, aggregates increased by approximately 2% in the buffer-only sample and the sample with polysorbate. Levels in samples containing either of the HPßCD excipients were lower. HPßCD was seen to reduce heat-induced aggregation in a concentration-dependent manner and at 100 mM was superior to trehalose (Figure 3).
Figure 3. Effects of KLEPTOSE® HP or HPB on heat-induced aggregation.
Conclusions
In this case study, the investigation of two commercially available HPßCD excipients confirmed their activities as surfactants in protein formulations. The anti-aggregation properties of both were confirmed using orthogonal methods (NanoDSF and SEC), showing them to:
- Be effective in reducing both agitation and thermal stress-induced aggregation.
- Prevent fragmentation during agitation.
- Reduce protein unfolding.
- This dual function stabilization property makes HPßCD a promising excipient to be explored for extending the shelf life of therapeutic proteins.
Case Study 2: Reverting Reversible Aggregates into Monomers and Arresting Rate of Aggregation of Bevacizumab
Monoclonal antibodies (mAbs) exhibit concentration-dependent self-association behavior. At high antibody concentrations, this may result in the formation of reversible aggregates. These aggregates may subsequently aggregate further into an irreversible state, which will ultimately reduce drug efficacy and safety. In addition, in downstream process, an increase in aggregate level is often observed during concentration and buffer exchange steps. This study tested the hypothesis that HPßCD can interact in such a way as to interfere with the self-association process, thereby reducing reversible aggregates, as well as reducing aggregate formation at buffer exchange and protein concentration step.
Bevacizumab (AVASTIN®), a monoclonal IgG1 antibody, was chosen as the model protein, as it is known to aggregate in both reversible and nonreversible aggregate forms. The commercial formulation of the drug contains 50 mM phosphate buffer at pH 6.2 and is supplemented with 60 mg/ml trehalose and 0.04% polysorbate 20. The experimental objective was to evaluate the effects of both the commercial HPßCD products on two aspects of bevacizumab aggregation: (i) during the buffer exchange and ultra-filtration steps that result in protein concentration, and (ii) maintaining formulation stability under stress conditions.
Buffer exchange and protein concentration
Buffer exchange and protein concentration steps were investigated using 50 mM phosphate elution buffer alone and elution buffer with the inclusion of 100 mM HPßCD. Each buffer solution was run through a Sephadex G-25 PD-10 desalting column loaded with bevacizumab in its original formulation. The resulting eluates were analyzed by SEC–HPLC to determine their aggregation profiles and were then concentrated further, from 9 mg/ml to 50 mg/ml, using an ultrafiltration centrifugal column with a 30 kD weight cut-off. These concentrated samples were also subjected to SEC–HPLC analysis. Aggregate levels were compared for the untreated sample, the sample after buffer exchange, and the sample following further concentration.
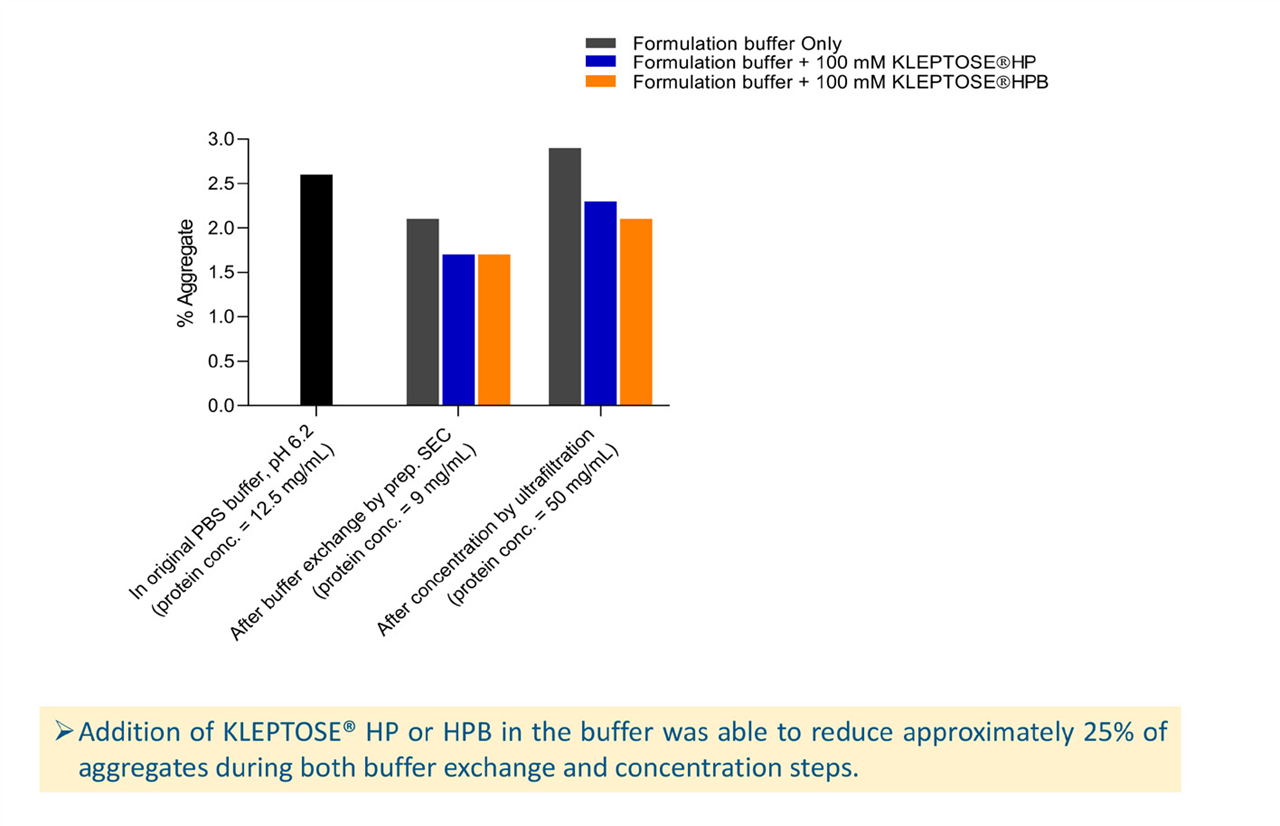
The results showed that aggregate levels actually reduced following the buffer exchange step (see Figure 4); this is possibly caused by dissociation of the reversible aggregate into two monomers and were lowest in the HPßCD-containing samples, suggesting that HPßCD can reverse reversible aggregation. After the concentration step, aggregate levels increased significantly for all three samples. Overall, the addition of HPßCD in the buffer reduced approximately 25% of aggregates during both buffer exchange and concentration steps.
Figure 4. Aggregation level after buffer exchange and concentration process.
Formulation stability
In this study, bevacizumab was formulated at a protein concentration of 25 mg/mL (the therapeutic-use level) in 50 mM sodium phosphate buffer pH 6.2, with varying concentrations of HPßCD (50, 100, and 200 mM) replacing the trehalose and polysorbate used in the commercial formulation. These formulations were compared with control formulations of: (i) buffer only and (ii) commercial formulation (CF). All formulations were prepared in triplicate. The stress conditions and analytical methods used to test formulation stability were as described in the first case study.
Examination of the aggregate levels in the samples immediately after formulation indicated that the presence of HPßCD reduced the level of reversible aggregates in a concentration-dependent manner. It was hypothesized that there could be interaction between bevacizumab and HPßCD, which might then interfere with the self-association of bevacizumab, thereby reducing the formation of reversible aggregates. Investigation of this is currently ongoing.
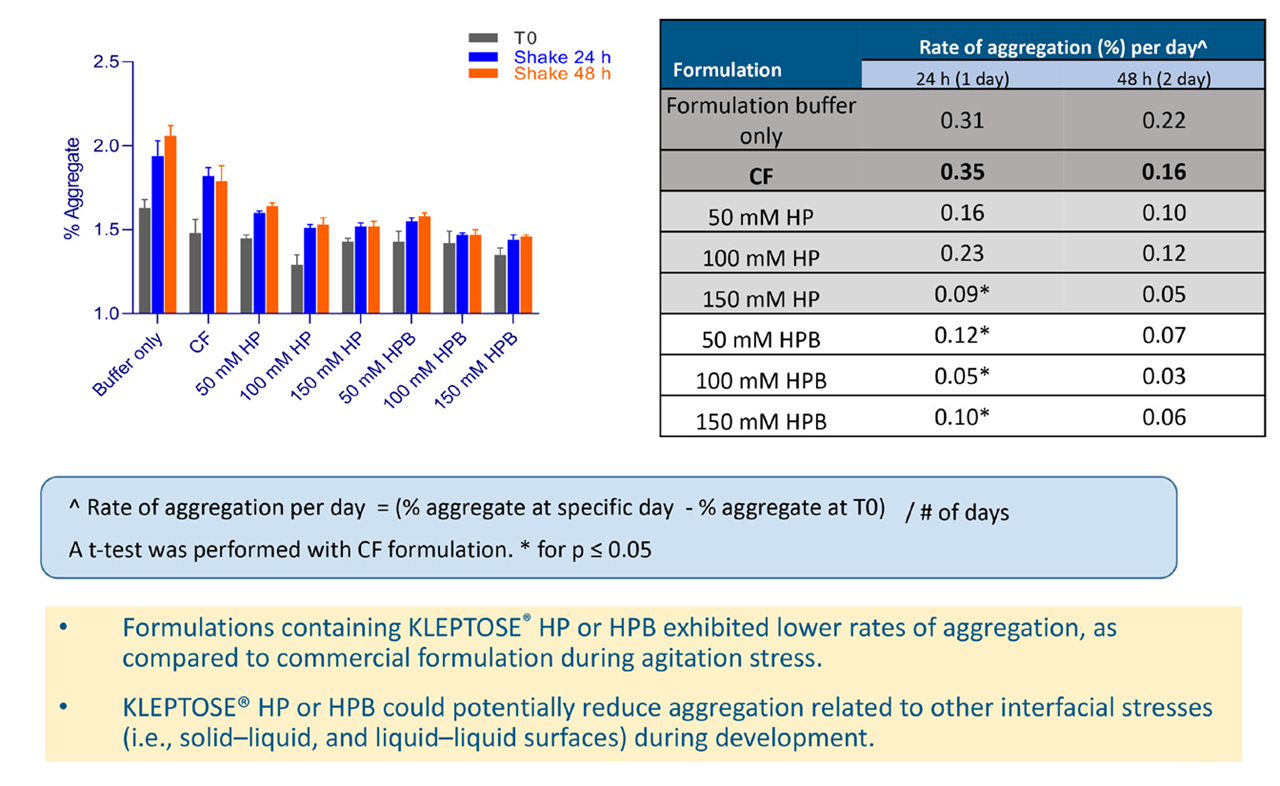
In the agitation studies, aggregate levels were analyzed at 0, 24, and 48 hours and showed a progressive increase for all samples. However, the rates of increase, calculated using the absolute increments of aggregate percentage divided by the number of stress days, differed (see Figure 5). HPßCD-supplemented formulations displayed slower aggregation rates compared with the commercial formulation. While rate differences appear low, they are likely to be significant over the course of long-term storage and affect the overall shelf life of the protein. Potentially, HPßCD could also be used to reduce aggregation related to other interfacial stresses, such as solid-liquid and liquid-liquid surfaces during drug development.
Figure 5. Aggregation of Avastin® after 24 and 48 hours of agitation.
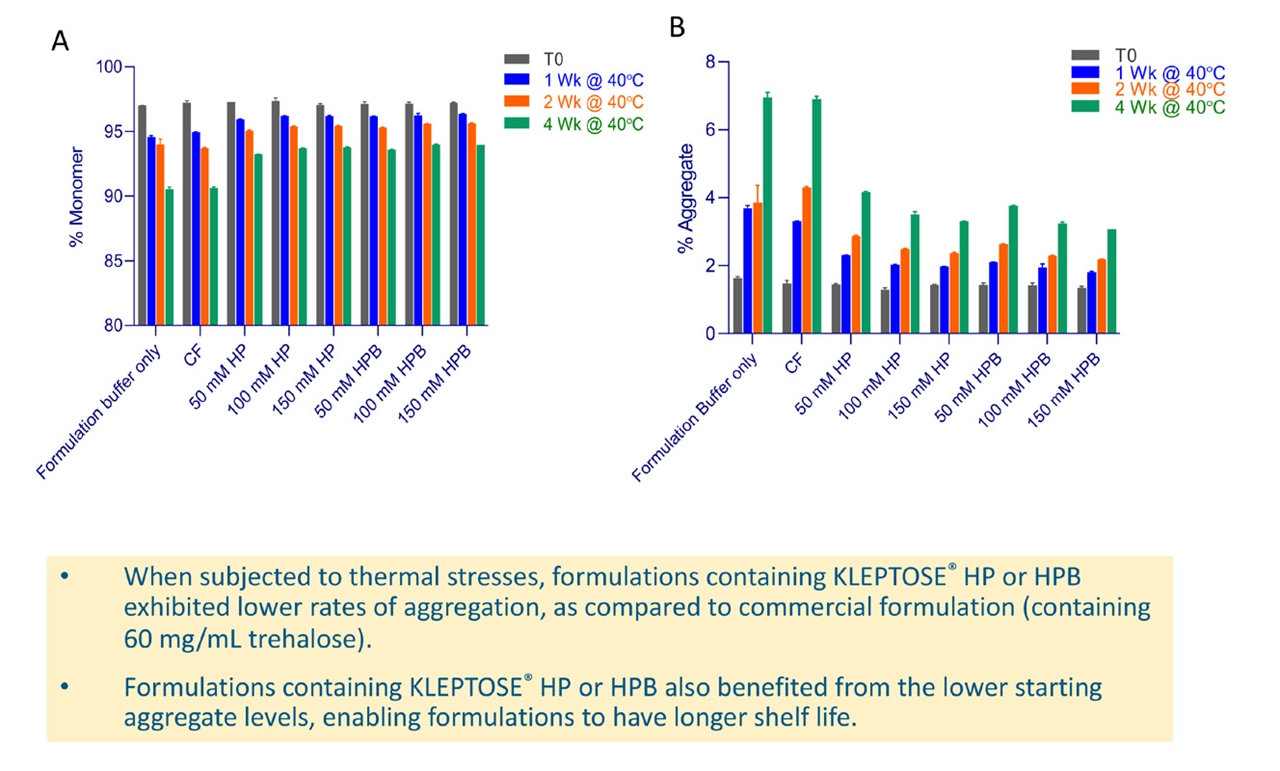
The protective effects of HPßCD were also noted when samples were subjected to thermal stress being held at 40°C and tested at 0, 1, 2 and 4 weeks (see Figure 6). Formulations containing HPßCD exhibited significantly lower rates of aggregation compared with the commercial formulation and also benefited from the lower starting aggregate levels, conferring a longer shelf life. Summarizing the data as rates of decrease in monomer shows that at 40]C HPßCD decreased the rate of degradation of bevacizumab by around half compared with that of the commercial formulation. Both forms of HPßCD tested were able to reduce rate of degradation in a concentration-dependent manner.
Figure 6. (A) % Monomer and (B) % aggregate of bevacizumab after incubation at 40°C for 1, 2, and 4 weeks.
Conclusions
The two forms of HPßCD studied were shown to reduce the formation of reversible aggregates in bevacizumab formulations. Both were beneficial in reducing aggregates in buffer exchange and concentration steps and were also effective in decreasing both agitation and thermal stress-induced aggregation. During thermal stress, the presence of HPßCD reduced the rate of degradation of bevacizumab by approximately half, as compared to commercial formulation. Potentially, this could extend the shelf life of bevacizumab formulations.
In Summary
Studies on the utility of HPβCD as a functional excipient in the liquid formulation of biologics indicate it to be a potential alternative to polysorbates for protein stabilization. The work reported here shows the effectiveness of HPβCD with model proteins in reducing both agitation and thermal stress-induced aggregation, as well as the rate of aggregation. HPβCD also prevented fragmentation during agitation and reduced protein unfolding. When tested with bevacizumab, HPßCD reduced the rate of degradation of the mAb by approximately half, as compared to commercial formulation. Since HPßCD is already approved for parenteral small-molecule applications, it holds considerable promise for the future of biologics formulation.
References
1. Serno, T., Geidobler, R & Winter, G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv Drug Deliv Rev, 2011. 63(13): p1086-106.
® Registered trademark(s) of Roquette Frères. The information contained in this document is to the best of our knowledge true and accurate but all instructions, recommendations or suggestions are made without any guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission. For questions about a product’s compliance with additional countries’ standards not listed above, please contact your local Roquette representative.
Avastin® is a registered trademark of Genentech, Inc.
Sporanox® is a registered trademark of Janssen Pharmaceutica NV
MitoExtra™ is a registered trademark of SuperGen Inc.