PEARLITOL® CR-H - HYPROMELLOSE - MANNITOL
PEARLITOL®
®Registered Trademark(s) of Roquette Frères
- Controlled release
- designed for direct compression
Co-processed Mannitol HPMC
Applications
- 1. Solid Forms - Tablets
- Swallowable Tablets
- 5. Other Solid Forms
- Losenges
Functional properties
- Modified Release Agents
- Sustained Release Agents
Documents
Product Specification Sheet
Name
Region
Size
Download
PEARLITOL® CR H - EXP
473,97 Ko
Region :
473,97 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
PEARLITOL® CR H - EXP
Europe, BE
EN
468,59 Ko
Region : Europe, BE
EN
468,59 Ko
PEARLITOL® CR H - EXP
Europe, CY
EN
466,15 Ko
Region : Europe, CY
EN
466,15 Ko
PEARLITOL® CR H - EXP
Europe, DE
EN
471,37 Ko
Region : Europe, DE
EN
471,37 Ko
PEARLITOL® CR H - EXP
Americas, CA
EN
249,49 Ko
Region : Americas, CA
EN
249,49 Ko
PEARLITOL® CR H - EXP
Europe, CH
EN
468,82 Ko
Region : Europe, CH
EN
468,82 Ko
PEARLITOL® CR H - EXP
Asia, CN
EN
289,44 Ko
Region : Asia, CN
EN
289,44 Ko
PEARLITOL® CR H
Europe, DK
EN
327,40 Ko
Region : Europe, DK
EN
327,40 Ko
PEARLITOL® CR H - EXP
Europe, AT
DE
476,88 Ko
Region : Europe, AT
DE
476,88 Ko
PEARLITOL® CR H - EXP
Europe, BE
DE
479,71 Ko
Region : Europe, BE
DE
479,71 Ko
PEARLITOL® CR H - EXP
Europe, BE
FR
577,42 Ko
Region : Europe, BE
FR
577,42 Ko
PEARLITOL® CR H - EXP
Europe, BE
NL
474,07 Ko
Region : Europe, BE
NL
474,07 Ko
PEARLITOL® CR H - EXP
Americas, BR
PT
484,42 Ko
Region : Americas, BR
PT
484,42 Ko
PEARLITOL® CR H - EXP
Europe, BG
BG
619,32 Ko
Region : Europe, BG
BG
619,32 Ko
PEARLITOL® CR H - EXP
Europe, CY
EL
609,66 Ko
Region : Europe, CY
EL
609,66 Ko
PEARLITOL® CR H - EXP
Europe, CY
TR
613,97 Ko
Region : Europe, CY
TR
613,97 Ko
PEARLITOL® CR H - EXP
Europe, DE
DE
484,04 Ko
Region : Europe, DE
DE
484,04 Ko
PEARLITOL® CR H - EXP
Americas, CA
FR
358,06 Ko
Region : Americas, CA
FR
358,06 Ko
PEARLITOL® CR H - EXP
Europe, CH
DE
479,82 Ko
Region : Europe, CH
DE
479,82 Ko
PEARLITOL® CR H - EXP
Europe, CH
FR
577,83 Ko
Region : Europe, CH
FR
577,83 Ko
PEARLITOL® CR H - EXP
Europe, CH
IT
565,42 Ko
Region : Europe, CH
IT
565,42 Ko
PEARLITOL® CR H - EXP
Asia, CN
ZH
504,96 Ko
Region : Asia, CN
ZH
504,96 Ko
PEARLITOL® CR H - EXP
Europe, CZ
CS
592,89 Ko
Region : Europe, CZ
CS
592,89 Ko
PEARLITOL® CR H - EXP
Europe, EE
ET
569,62 Ko
Region : Europe, EE
ET
569,62 Ko
PEARLITOL® CR H - EXP
Europe, DK
DA
476,14 Ko
Region : Europe, DK
DA
476,14 Ko
PEARLITOL® CR H - EXP
Europe, ES
ES
480,17 Ko
Region : Europe, ES
ES
480,17 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
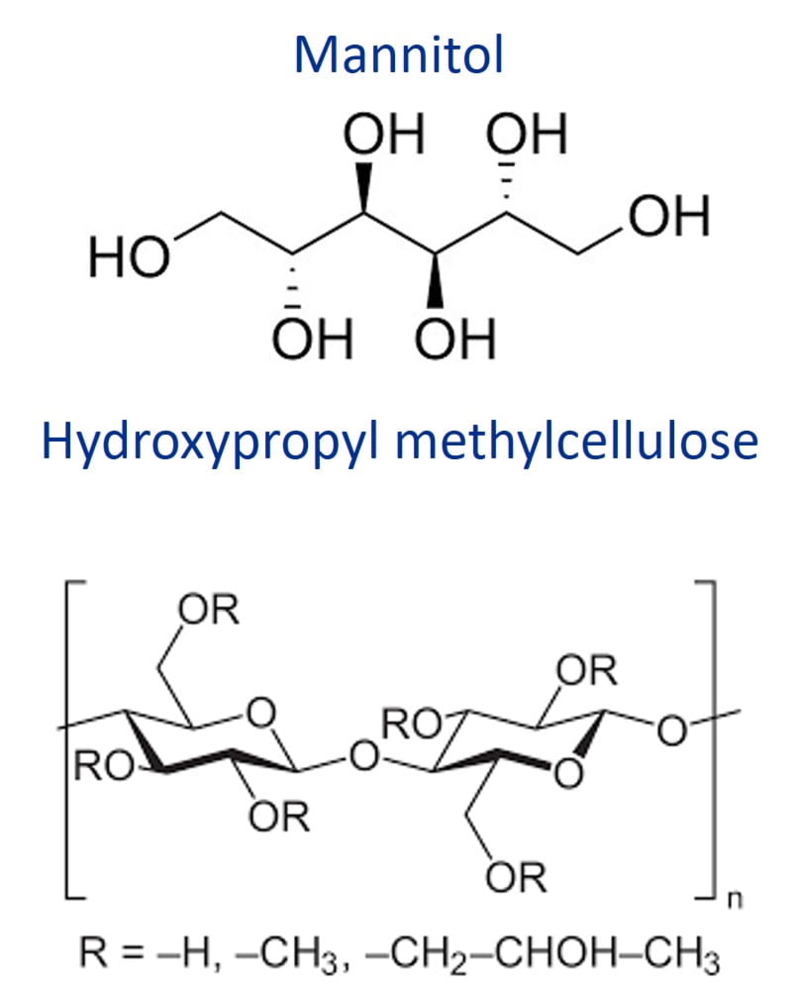
| Synonyms | D-Mannitol-Hydroxypropyl methylcellulose, HPMC |
|---|---|
| Composition | D-Mannitol 30%-HPMC K4M 70% |
| CAS number | D-Mannitol 69-65-8 Hypromellose 9004-65-3 |
| Physical form or apperance | White to yellowish-white, powder or granules |
| Application | PEARLITOL® CR-H co-processed mannitol HPMC is a direct compression excipient providing functional properties of binder as well as controlled release to your tablet. PEARLITOL® CR-H can be used in both pharmaceutical and nutraceutical oral dosage forms. |
| Teste/Odor | Neutral taste, slightly sweet |
| Morphology |
|
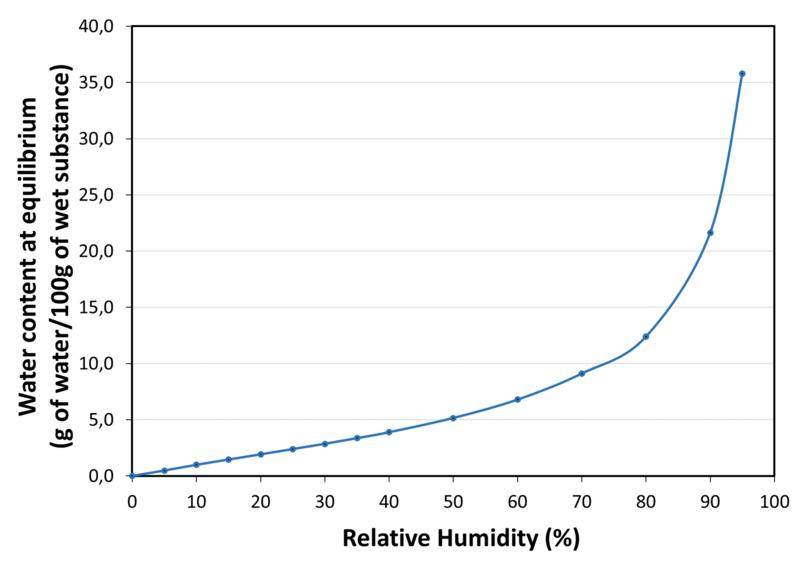
| Water sorption isotherm at 20°C |
|
| Chemical Structure |
|
| Average molecular weight | Mannitol: 182.2 g/mol |
| Maximal Water content (LOD) | 4.00 |
| Solubility | Partially soluble in cold water. |
| Minimum melting temperature | Mannitol: 165 °C |
| Maximum melting temperature | Mannitol: 170 °C |
| Average mean particle diameter | 160 |
| dv10 Particle size distribution | 60 |
| dv50 Particle size distribution | 140 |
| dv90 Particle size distribution | 290 |
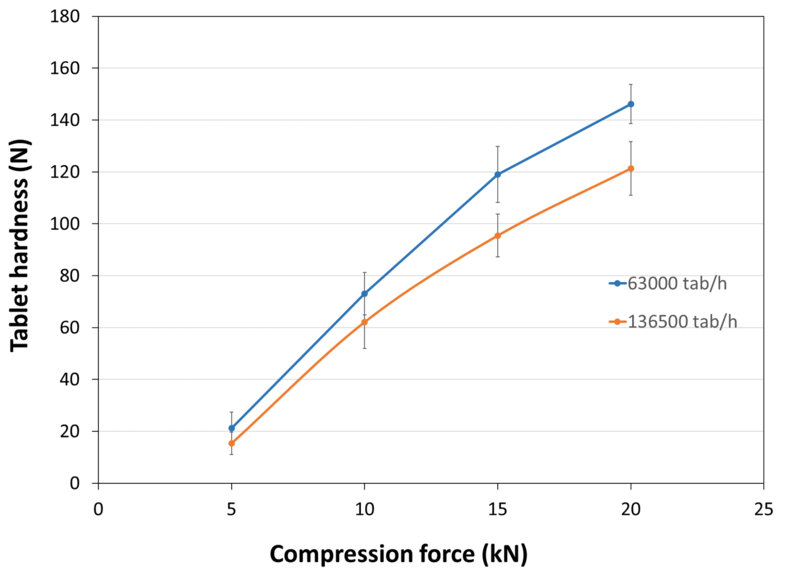
| Tablet Hardness |
|
| Experimental Conditions for Compression Behavior_Tablet Press | STYL'ONE EVO |
| Experimental Conditions for Compression Behavior_Production Speed | 30 and 65 RPM (linear punch velocity: 49 and 91 mm/s; simulated KORSCH XL-400 rotary press speed: 63,000 and 136,500 tablets/hour) |
| Experimental Conditions for Compression Behavior_Tooling | Diameter 11.28 mm flat |
| Experimental Conditions for Compression Behavior_Formuls | PEARLITOL® CR H / external lubrication with magnesium stearate |
| Experimental Conditions for Compression Behavior_Tablet Mass | 400 mg |
| Powder Flowability (according to Ph.Eur. 2.9.16, 10mm outflow opening) | 12 |
| Bulk Density (g/ml) | 0.34 |
| Tapped Density (g/ml) | 0.43 |
| True Density (g/ml) | 1.46 |
| Specific Surface Area (m²/g) | 0.80 |
| Angle of Repose (°) | 36 |
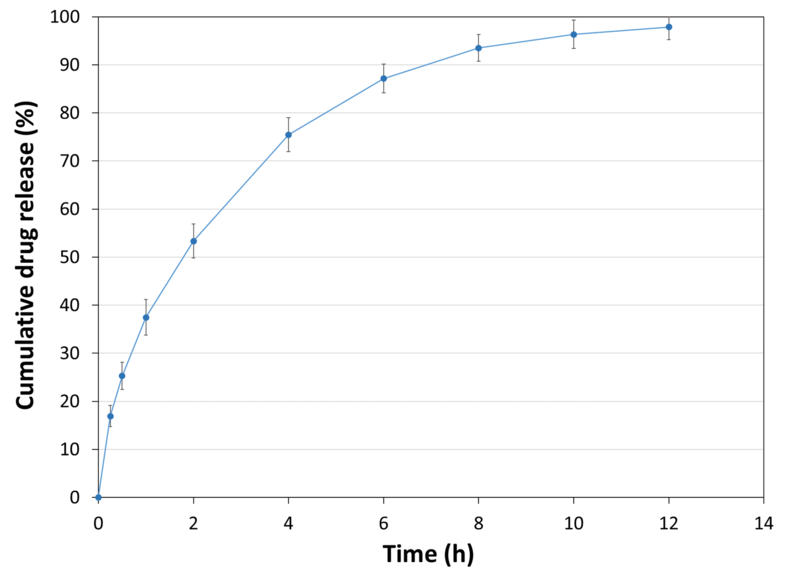
| API Release Kinetics |
|
| Experimental Conditions for Sustained Release Behaviour_Tablet Press | STYL'ONE EVO |
| Experimental Conditions for Sustained Release Behaviour_Production Speed | 30 RPM (linear punch velocity: 49 mm/s; simulated KORSCH XL-400 rotary press speed: 63,000 tablets/hour) |
| Experimental Conditions for Sustained Release Behaviour_Tooling | Diameter 13 mm flat |
| Experimental Conditions for Sustained Release Behaviour_Formula | 50% Metformin / 49% PEARLITOL® CR-H / 1% magnesium stearate |
| Experimental Conditions for Sustained Release Behaviour_Tablet Mass | 1,000 mg |
| Experimental Conditions for Sustained Release Behaviour_Tablet Hardness | 109 N |
| Experimental Conditions for Sustained Release Behaviour_Tablet Thickness | 6.8 mm |
| Experimental Conditions for Sustained Release Behaviour_Dissolution Bath | USP II with helical sinker |
| Experimental Conditions for Sustained Release Behaviour_Release Media | pH transition method adapted from USP <711> Dissolution Method A |