LYCATAB® C - PREGELATINIZED MAIZE STARCH
LYCATAB®
®Registered Trademark(s) of Roquette Frères
- The excipient with the fastest disintegration, providing higher productivity
- Adapted to hard capsule filling
Partially pregelatinized starch
Applications
- 1. Solid Forms - Tablets
- Swallowable Tablets
- 2. Solid Forms - Capsules
- Hard Capsules Fill
- 5. Other Solid Forms
- Stick Packs and sachets
Functional properties
- Formulations Aids
- Disintegrants & Super Disintegrants
- Fillers and Binders for Direct Compression
- Fillers and Binders for Roller Compaction
Physical and chemical properties
- Formulation & Manufacturing
- Consistent drug dissolution, uniform weight
- Physical Characteristics
- Easy-flowing powder
- General Properties
- Label-friendly
- Multicompendial
- Stability & Compliance
- pH-independent disintegration
Documents
Product Specification Sheet
Name
Region
Size
Download
LYCATAB® C
469,49 Ko
Region :
469,49 Ko
LYCATAB® C
475,42 Ko
Region :
475,42 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
LYCATAB® C
Europe, BE
EN
502,29 Ko
Region : Europe, BE
EN
502,29 Ko
LYCATAB® C
Europe, CH
EN
502,46 Ko
Region : Europe, CH
EN
502,46 Ko
LYCATAB® C
Asia, CN
EN
315,98 Ko
Region : Asia, CN
EN
315,98 Ko
LYCATAB® C
Europe, DE
EN
505,29 Ko
Region : Europe, DE
EN
505,29 Ko
LYCATAB® C
Europe, FI
EN
502,26 Ko
Region : Europe, FI
EN
502,26 Ko
LYCATAB® C
Europe, FR
EN
502,62 Ko
Region : Europe, FR
EN
502,62 Ko
LYCATAB® C
Europe, GB
EN
365,15 Ko
Region : Europe, GB
EN
365,15 Ko
LYCATAB® C
Americas, Asia, Oceania, Africa
EN
259,28 Ko
Region : Americas, Asia, Oceania, Africa
EN
259,28 Ko
LYCATAB® C
Europe, AT
DE
510,89 Ko
Region : Europe, AT
DE
510,89 Ko
LYCATAB® C
Europe, BE
DE
513,60 Ko
Region : Europe, BE
DE
513,60 Ko
LYCATAB® C
Europe, BE
FR
613,47 Ko
Region : Europe, BE
FR
613,47 Ko
LYCATAB® C
Europe, BE
NL
510,19 Ko
Region : Europe, BE
NL
510,19 Ko
LYCATAB® C
Americas, BR
PT
524,38 Ko
Region : Americas, BR
PT
524,38 Ko
LYCATAB® C
Europe, CH
DE
513,87 Ko
Region : Europe, CH
DE
513,87 Ko
LYCATAB® C
Europe, CH
FR
613,99 Ko
Region : Europe, CH
FR
613,99 Ko
LYCATAB® C
Europe, CH
IT
603,39 Ko
Region : Europe, CH
IT
603,39 Ko
LYCATAB® C
Asia, CN
ZH
535,31 Ko
Region : Asia, CN
ZH
535,31 Ko
LYCATAB® C
Europe, CZ
CS
630,59 Ko
Region : Europe, CZ
CS
630,59 Ko
LYCATAB® C
Europe, DE
DE
516,64 Ko
Region : Europe, DE
DE
516,64 Ko
LYCATAB® C
Europe, ES
ES
516,48 Ko
Region : Europe, ES
ES
516,48 Ko
LYCATAB® C
Europe, DK
DA
510,29 Ko
Region : Europe, DK
DA
510,29 Ko
LYCATAB® C
Europe, FI
FI
516,53 Ko
Region : Europe, FI
FI
516,53 Ko
LYCATAB® C
Europe, FR
FR
613,69 Ko
Region : Europe, FR
FR
613,69 Ko
LYCATAB® C
Americas, Asia, Oceania, Africa
ES
271,63 Ko
Region : Americas, Asia, Oceania, Africa
ES
271,63 Ko
LYCATAB® C
Americas, Asia, Oceania, Africa
PT
276,44 Ko
Region : Americas, Asia, Oceania, Africa
PT
276,44 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
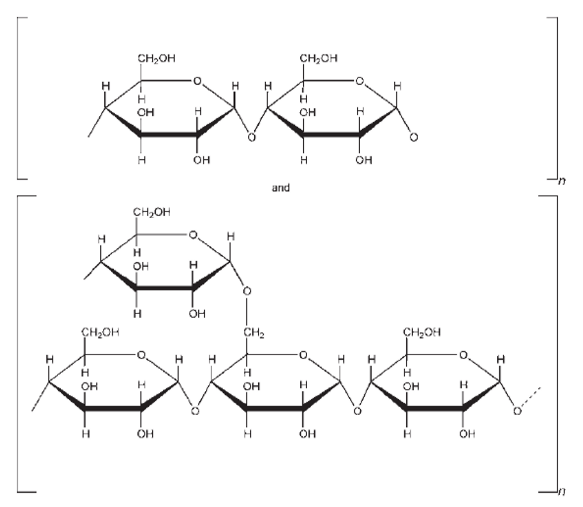
| Synonyms | Pregelatinized Starch |
|---|---|
| CAS number | 9005-25-8 |
| Physical form or apperance | White or almost white powder |
| Application | LYCATAB® C partially pregelatinized starch is a direct compression excipient with filler/binder and disintegrant properties. It is used both in nutraceutical and pharmaceutical oral dosage forms, mainly for swallowable tablets and hard capsules. |
| Source | Maize |
| Teste/Odor | Odorless powder |
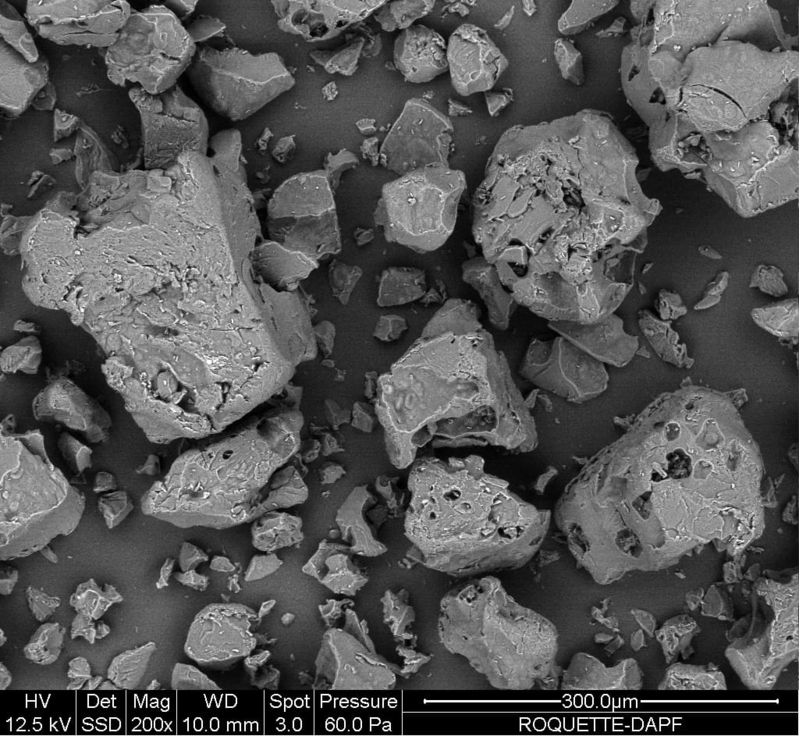
| Morphology |
|
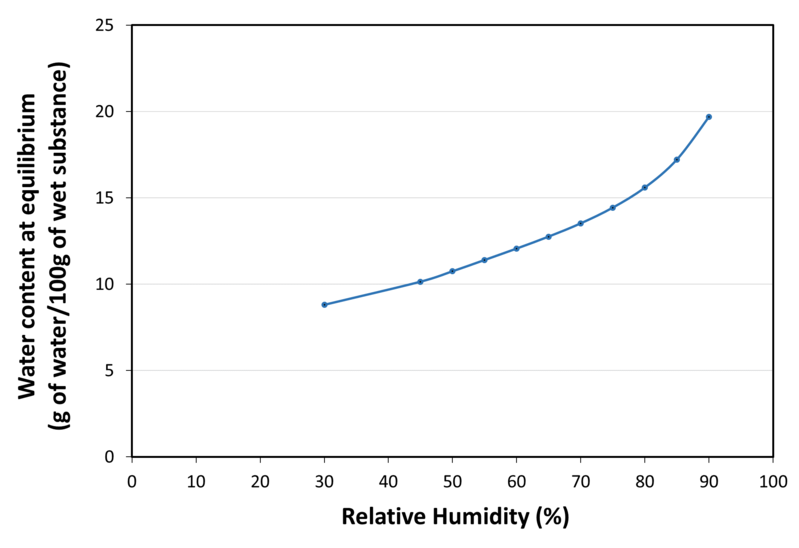
| Water sorption isotherm at 20°C |
|
| Chemical Structure |
|
| Maximal Water content (LOD) | 14.00 |
| Solubility | Swells in cold water, practically insoluble in organic solvents |
| Average mean particle diameter | 90 |
| dv10 Particle size distribution | 35 |
| dv50 Particle size distribution | 90 |
| dv90 Particle size distribution | 166 |
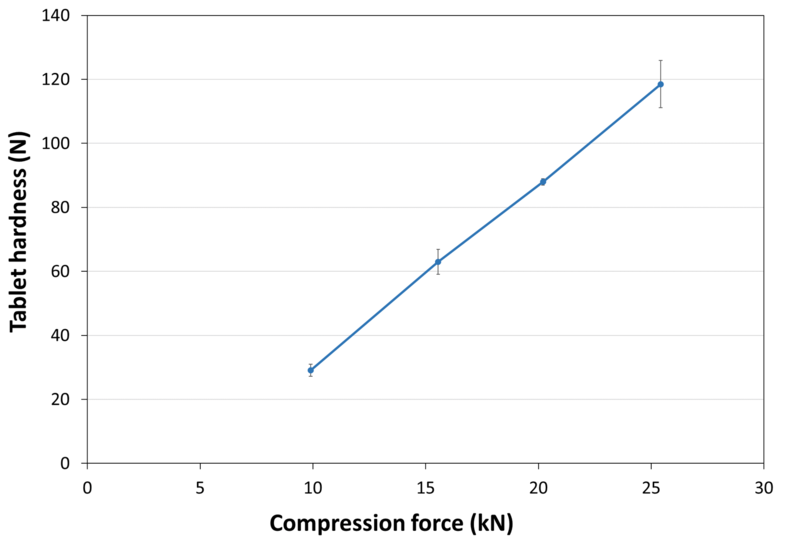
| Tablet Hardness |
|
| Experimental Conditions for Compression Behavior_Tablet Press | KORSCH XP 1 |
| Experimental Conditions for Compression Behavior_Production Speed | 20 tablets/min |
| Experimental Conditions for Compression Behavior_Tooling | Diameter 13 mm R13 concave |
| Experimental Conditions for Compression Behavior_Formuls | 100% LYCATAB® C |
| Experimental Conditions for Compression Behavior_Tablet Mass | 600 mg |
| Powder Flowability (according to Ph.Eur. 2.9.16, 10mm outflow opening) | 5 |
| Bulk Density (g/ml) | 0.63 |
| Tapped Density (g/ml) | 0.81 |
| True Density (g/ml) | 1.52 |
| Specific Surface Area (m²/g) | 0.30 |
| Angle of Repose (°) | 30 |