ANHYDROUS DEXTROSE BIOPHARMA
- Very high purity for application in BioPharma
High purity dextrose
Applications
- Upstream
- Single-use Manufacturing
Functional properties
- Cell Culture Ingredients
- Precision Dispense
Physical and chemical properties
- White or almost white, crystalline powder
Documents
Product Specification Sheet
Name
Region
Size
Download
DEXTROSE ANHYDROUS BIOPHARMA
485,37 Ko
Region :
485,37 Ko
DEXTROSE ANHYDROUS BIOPHARMA
466,85 Ko
Region :
466,85 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
DEXTROSE ANHYDROUS BIOPHARMA
Europe, CH
EN
515,61 Ko
Region : Europe, CH
EN
515,61 Ko
DEXTROSE ANHYDROUS BioPharma
Europe, DE
EN
519,52 Ko
Region : Europe, DE
EN
519,52 Ko
DEXTROSE ANHYDROUS BioPharma
Europe, FR
EN
516,62 Ko
Region : Europe, FR
EN
516,62 Ko
DEXTROSE ANHYDROUS BioPharma
Europe, GB
EN
377,71 Ko
Region : Europe, GB
EN
377,71 Ko
DEXTROSE ANHYDROUS BioPharma
Americas, Asia, Oceania, Africa
EN
271,62 Ko
Region : Americas, Asia, Oceania, Africa
EN
271,62 Ko
DEXTROSE ANHYDROUS BIOPHARMA
Europe, IE
EN
516,43 Ko
Region : Europe, IE
EN
516,43 Ko
DEXTROSE ANHYDROUS BIOPHARMA
Europe, IT
EN
519,53 Ko
Region : Europe, IT
EN
519,53 Ko
DEXTROSE ANHYDROUS BioPharma
Americas, US
EN
330,80 Ko
Region : Americas, US
EN
330,80 Ko
DEXTROSE ANHYDROUS BioPharma
Asia, SG
EN
272,98 Ko
Region : Asia, SG
EN
272,98 Ko
DEXTROSE ANHYDROUS BIOPHARMA
Europe, CH
DE
528,44 Ko
Region : Europe, CH
DE
528,44 Ko
DEXTROSE ANHYDROUS BIOPHARMA
Europe, CH
FR
627,45 Ko
Region : Europe, CH
FR
627,45 Ko
DEXTROSE ANHYDROUS BIOPHARMA
Europe, CH
IT
616,16 Ko
Region : Europe, CH
IT
616,16 Ko
DEXTROSE ANHYDROUS BioPharma
Europe, DE
DE
532,36 Ko
Region : Europe, DE
DE
532,36 Ko
DEXTROSE ANHYDROUS BioPharma
Europe, ES
ES
531,39 Ko
Region : Europe, ES
ES
531,39 Ko
DEXTROSE ANHYDROUS BioPharma
Europe, FR
FR
628,99 Ko
Region : Europe, FR
FR
628,99 Ko
DEXTROSE ANHYDROUS BioPharma
Americas, Asia, Oceania, Africa
ES
283,27 Ko
Region : Americas, Asia, Oceania, Africa
ES
283,27 Ko
DEXTROSE ANHYDROUS BioPharma
Americas, Asia, Oceania, Africa
PT
288,85 Ko
Region : Americas, Asia, Oceania, Africa
PT
288,85 Ko
DEXTROSE ANHYDROUS BioPharma
Americas, Asia, Oceania, Africa
RU
415,65 Ko
Region : Americas, Asia, Oceania, Africa
RU
415,65 Ko
DEXTROSE ANHYDROUS BIOPHARMA
Europe, IT
IT
619,82 Ko
Region : Europe, IT
IT
619,82 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Synonyms | Glucose Anhydrous, Purified Glucose |
|---|---|
| CAS number | 50-99-7 |
| Physical form or apperance | White or almost white, crystalline powder |
| Application | Dextrose Anhydrous BioPharma is used upstream in the cell culture process as an energy and carbon source during the production of therapeutic proteins. |
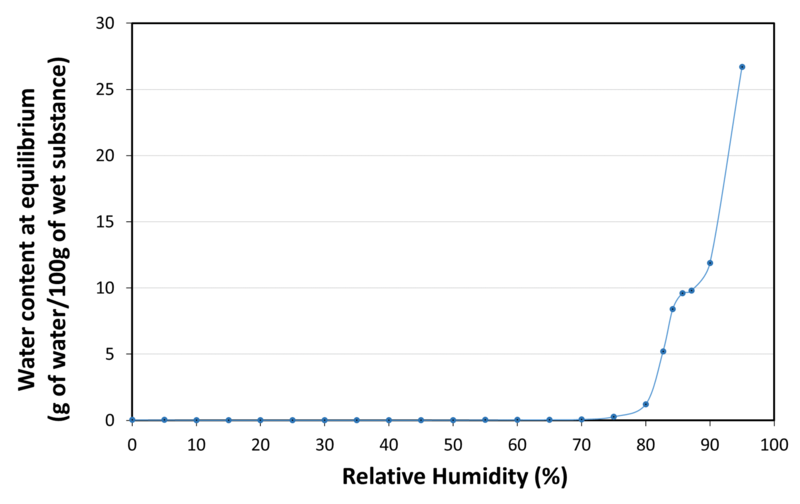
| Water sorption isotherm at 20°C |
|
| Chemical Structure |
|
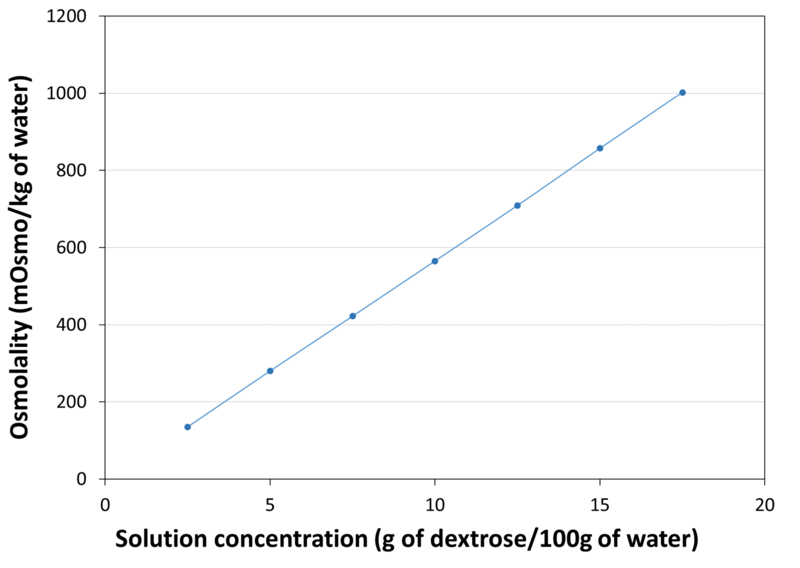
| Osmolality |
|
| Average molecular weight | 180.2 g/mol |
| Maximal Water content (LOD) | 1.00 |
| Solubility | Freely soluble in water (83 g/100 ml of water at 20°C), very slightly soluble in ethanol (96%) |
| Minimum melting temperature | 145 °C |
| Maximum melting temperature | 150 °C |
| Glass Transition Temperature of the Cryo-Concentrated Phase | -45.00 |