Viral Inactivation in Bioprocessing Using Hydroxypropyl Beta-Cyclodextrins, a Compendial Excipient
Published January 27, 2026
Presented at the AAPS 2024 PharmaSci 360, October 20-23, Salt Lake City, Utah, USA and at the AAPS 2025 PharmaSci 360, November 9-12, San Antonio, Texas, USA.
Authors
Shiqi HONG
Biopharma Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Tao Peng
BioPharma Research Manager, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Hailong Zhang
Analytical Manager, R&D analysis, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Lucas Goh
Biopharma Team Lead, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Jiayi Huang
Biopharma Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Keat Theng Chow
Pharmaceutical Research Manager, Pharmaceutical R&D, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Deepak Bahl
Global Head of Applied Science - Pharma, Roquette Philadelphia
PURPOSE
Virus inactivation is an essential step in the purification of biologics (mAbs, viral vectors, blood products, etc.) derived from cell expression systems, plasma fractionation, and other sources. Conventionally, S/D (solvent/detergent) is widely applied to inactive enveloped viruses, among which TritonTM X-100 is the most used detergent. However, due to its environmental risk, TritonTM X-100 has been banned in Europe.1 The industry has been searching for suitable replacements for TritonTM X-100.2
This study aims to validate the virus inactivation efficiency of hydroxypropyl beta-cyclodextrin (HPβCD) when applied to the harvested cell culture fluid (HCCF). The compatibility of HPβCD in subsequent downstream processes will also be investigated.
MATERIALS AND METHODS
Hydroxypropyl beta-cyclodextrin (KLEPTOSE® HP Biopharma) was obtained from Roquette Frères (Lestrem, France). Tri-n-butyl phosphate (TNBP) and TritonTM X-100 were sourced from Merck KGaA. The HCCF, protein A eluate of mAb X, and model viruses – xenotropic murine leukemia virus (X-MuLV) and herpes simplex virus type 1 (HSV-1) – were provided by Syngene International. Ipilimumab was produced in-house using the stable CHO cell line.
To evaluate virus inactivation efficiency, the HCCF of mAb X was spiked with 5% X-MuLV or HSV-1. Subsequently, the virus inactivation reagents, including different concentrations of HPβCD, 1% polysorbate, 1% TritonTM X-100, and combinations thereof with 0.3% TNBP, were added and incubated for a defined period of time. These samples were tested along with load and processing controls (i.e., media spiked with the virus but without virus inactivation reagents) using a cell-based infectivity assay. The virus titers were quantified, and log10 reduction values (LRV) were calculated statistically.
The compatibility of HPβCD with downstream processes was evaluated by assessing protein stability and impurity removal efficiency. HCCF of ipilimumab, spiked with 5% or 10% HPβCD, was subjected to protein A chromatography, followed by anion exchange chromatography (AIEX) and cation exchange chromatography (CIEX). The size and charge profiles of ipilimumab after each step were assessed using size exclusion chromatography (SEC-HPLC) and cation exchange chromatography (CIEX-HPLC). The host cell protein (HCP), host cell DNA (HCDNA), and leached protein A in the same samples were measured using an ELISA kit and Q-PCR assay, respectively. The remaining HPβCD in the protein sample after each chromatography step was also evaluated by C18 column equipped on HPLC with a corona charged aerosol detector (HPLC-CAD) and LC-mass spectrometer (LC-MS).
RESULTS
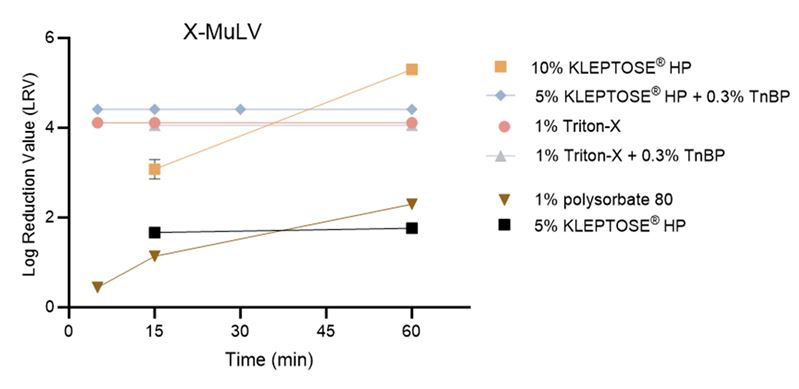
KLEPTOSE® HP is capable of extracting cholesterol and other lipids from the viral envelope, thereby disrupting the viral structure and leading to virus inactivation. The virus inactivation effect of KLEPTOSE® HP is concentration dependent. In the X-MuLV inactivation study (Figure 1), a 5% HPβCD solution resulted in a relatively modest virus inactivation, achieving approximately a 1.7 log₁₀ reduction at both 15 and 60 minutes, comparable to the reduction observed with 1% polysorbate. In contrast, a 10% KLEPTOSE® HP solution, as well as 5% KLEPTOSE® HP in combination with 0.3% TNBP achieved greater than 4 log₁₀ reduction, comparable to the reductions observed with 1% TritonTM X-100, either alone or in combination with 0.3% TNBP.
Figure 1. The inactivation of X-MuLV by different reagents in harvested cell culture fluid (HCCF).
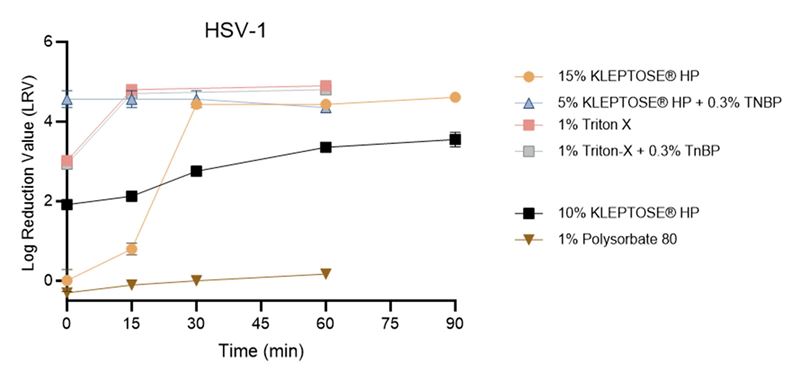
In the HSV-1 inactivation study (Figure 2), a 15% KLEPTOSE® HP solution achieved a greater than 4 log₁₀ reduction within 30 minutes. Furthermore, similar to the results observed with X-MuLV, the combination of 5% KLEPTOSE® HP with 0.3% TNBP, as well as 1% TritonTM X-100 alone or in combination with 0.3% TNBP, resulted in a rapid reduction exceeding 4 log₁₀.
Figure 2. The inactivation of HSV-1 by different reagents in harvested cell culture fluid (HCCF).
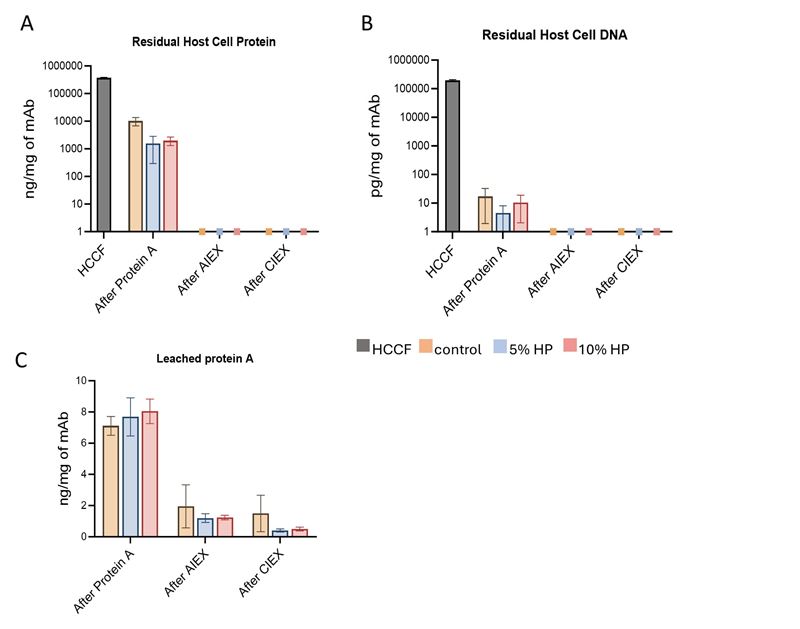
Additionally, HPβCD is fully compatible with downstream processes and offers the advantage of easy handling, as it is a highly soluble powder that, once dissolved, can be pumped without foam formation. The data indicate that the size and charge profiles of purified ipilimumab remain consistent across all samples, regardless of whether 5% or 10% KLEPTOSE® HP was added to the HCCF. The removal efficiency for impurities, including HCP, HCDNA, and leaked protein A is also comparable among the test samples (Figure 3).
Figure 3. Impurities removal of (A) host cell protein, (B) host cell DNA, and (C) leached protein A after each chromatography step for the starting HCCF load with different concentrations of HPβCD (KLEPTOSE® HP Biopharma).
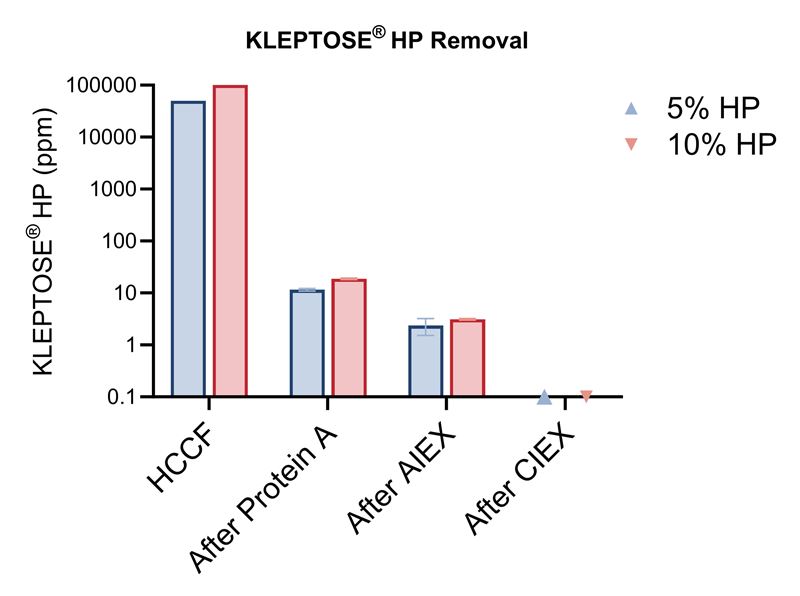
Moreover, HPβCD can be progressively removed during downstream processing. Protein A chromatography reduced the KLEPTOSE® HP concentration from the initial 50,000 ppm and 100,000 ppm to approximately 10 ppm, which was further decreased to below the quantitation limit (125 ppb) after the AIEX and CIEX steps (Figure 4).
Figure 4. The amount of remaining HPβCD (KLEPTOSE® HP Biopharma) in protein sample after each chromatography step.
Conclusion
HPβCD is an effective virus-inactivation agent that ensures the viral safety of biologics. It is compatible with downstream processes, maintaining product stability and purity. Given its established safety for parenteral administration – demonstrated by its inclusion in numerous approved drugs – HPβCD can potentially remain in the final drug formulation as a stabilizer. Moreover, multiple studies have shown that HPβCD protects biologics from the stresses encountered during various manufacturing steps, further supporting its role as a stabilizing agent.
References
1. Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006. EUR-Lex 1 May 2022; https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20220501.
2. Luo Wen et al. “Identification and characterization of a triton X-100 replacement for virus inactivation.” Biotechnol Prog. vol.36,6 (2020): e3036 https://doi.org/10.1002/btpr.3036