Tablet powder mix computational physics requirements to be processed by direct compression (DC)
Published January 27, 2026
Presented at the 13th world Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, March 28-31, 2022, Rotterdam, The Netherlands
Authors
Nicolas Descamps
Manager of the Functional Properties Analytical Laboratory, Roquette R&D
Philippe Lefèvre
Head of Global Pharma Applications Labs, Roquette
Dr Carmen Popescu
Global Technical Applications Specialist, Roquette
Introduction
Tablets, as a dosage form, have many advantages, like high patient compliance, portability, relatively low cost, and convenient scaling-up. Based on experimental study and quantitative evaluation, there is a need to find reliable computational physics criteria to qualify tablet powder mix (API and excipients) suitability for direct compression (DC). As literature suggests(1,2,3), the easiest way is to create a spider diagram based on measured physical powder parameters in acceptable range of values and define the conversion to radii from 0 to 10 scale.
Materials and Methods
Materials
Sitagliptin is used along with diet and exercise and sometimes with other medications to lower blood sugar levels in adults with type 2 diabetes. PEARLITOL® 200 SD mannitol and MICROCEL® MC-200 microcrystalline cellulose (MCC) are commonly used filler/binder in DC.
Methods
Bulk Densities (Db) (associated to die filling parameter): measured as per European Pharmacopeia 2.9.34, 𝐷𝑏=𝑚/𝑉𝑏.
Tapped density (Dt) (linked to flowability): measured as per European Pharmacopeia 2.9.34, 𝐷𝑡=𝑚/𝑉𝑡.
Hausner ratio (HR) (linked to flowability): as per European Pharmacopeia 2.9.34; 𝐻𝑅=𝐷𝑡/𝐷𝑏.
Angle of repose (linked to flowability): evaluated as per European Pharmacopeia 2.9.36.
Powder flow time (linked to flowability): measured as per European Pharmacopeia 2.9.16.
D10 (linked to flowability): The portion of particles with diameters smaller than D10 is 10% of the total volume of powder. Measured using a laser diffraction particle size analyzer.
Bulk density difference (∆Db) (powder mixture homogeneity): Calculated using minimum and maximum bulk densities of the individual components of the powder blend. Minor components (lubricant, glidant or flavor) are not considered for the bulk density difference calculation.
∆D𝑏=(𝐷(𝑏, 𝑚𝑎𝑥)−𝐷 (𝑏, 𝑚𝑖𝑛) )/𝐷(𝑏, 𝑚𝑖𝑛𝑖)
Particle size difference (∆D50) (powder mixture homogeneity): Calculated using minimum and maximum D50 of the individual components of the powder blend. Minor components (lubricant, glidant or flavor) are not considered for the particle size difference calculation.
∆D50 =(𝐷(50,𝑚𝑎𝑥)−𝐷(50,𝑚𝑖𝑛))/𝐷(50,𝑚𝑖𝑛)
Specific surface area (associated with compactibility): evaluated as per European Pharmacopeia 2.9.26.
Maximum tensile strength at P≤200MPa (𝜎𝑡) (associated with compactibility): as per European Pharmacopeia 2.9.8. Calculated using maximum hardness measured for a compression pressure P≤200 MPa. Measurement conditions: low speed (10tabs/min) with flat punch 10 mm diameter.
𝜎𝑡=2𝐻/(𝜋𝑑T𝑡)
with H the tablet hardness, dT the tablet diameter and t the tablet thickness.
Loss on drying (associated with stability): as per European Pharmacopeia 2.2.32.
Hygroscopicity (associated with stability): measured using a dynamic vapor sorption (DVS) analyzer as percentage increase in sample weight after being equilibrated at a relative humidity of 76% (+/-2%) and a temperature of 20°C.
RESULTS
We have selected 11 parameters under five categories; each category corresponds to an important step in the overall powder tabletability (figure 1).
Figure 1. Parameters influencing the overall powder tabletability grouped in five categories, related either to the processability or to the quality of the formula.
For each parameter, an acceptable range of values is defined and is expressed on a 0 to 10 scale. The measured values (v) and calculated radii are shown in table 1.
Table 1. Definition of the acceptable range of values (v) for each parameter. The factor to express the value on a 0 to 10 scale in indicated.
Prevalence | Parameter | Acceptable range of value (v) | Factor applied to v |
Powder mixture homogeneity* | Bulk density difference | 2 - 0 (-) | 10-5v |
| Particle size difference | 10 - 0 (-) | 10-v | |
Die filling | Bulk density | 0 - 0.8 (g/ml) | 12.5v |
Flowability | D10 | 0 - 100 ( | v/10 |
| Hausner radio | 2 - 1 (-) | (20-10v)/2 | |
| Angle of repose | 65 - 25 (°) | (650-10v)/40 | |
| Powder flow time | 20 - 3 (s) | (200-10v)/17 | |
| Compactibility | Specific surface area | 0 - 2.5 (m2/g) | 4v |
| Mac tablet tensile | 0.567 - 7 MPa | 4*Ln(v)+2.5 | |
Stability | Loss on drying | 10 - 0 (%) | 10-v |
| Hygroscopicity | 20 - 0 (%) | 10-(v/2) |
* Only valid for powder mixtures
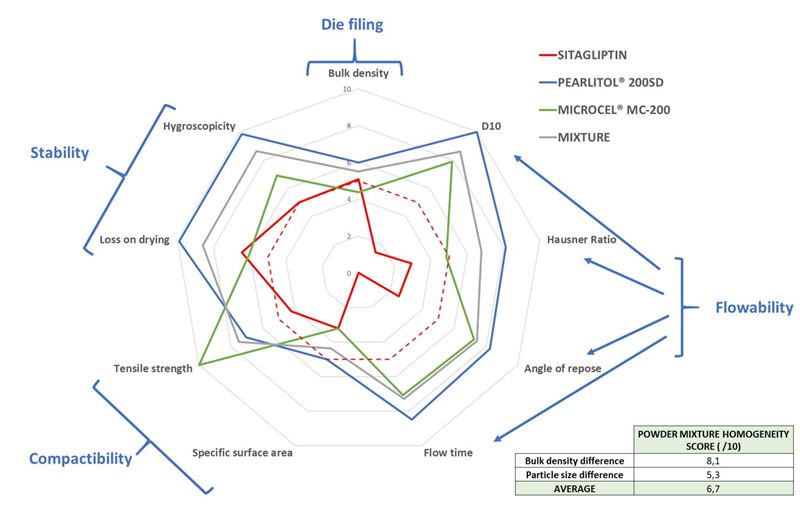
After conversion on a 0 to 10 scale according to factors presented in table 1, each component of a powder mixture can be represented on a spider plot (figure 2). Depending on the mass ratio of the components, the behavior of the mixture can be calculated (grey line in figure 2).
Figure 2. Spider plot representation of single powders parameters and predicted behavior of the mixture of those powders (composition of the mix: 10% Sitagliptin, 65% PEARLITOL® 200 SD, 25% MICROCEL® MC-200). Predicted mean score for mixture = 7.1/10. Bottom right table indicated the homogeneity score of the mixture.
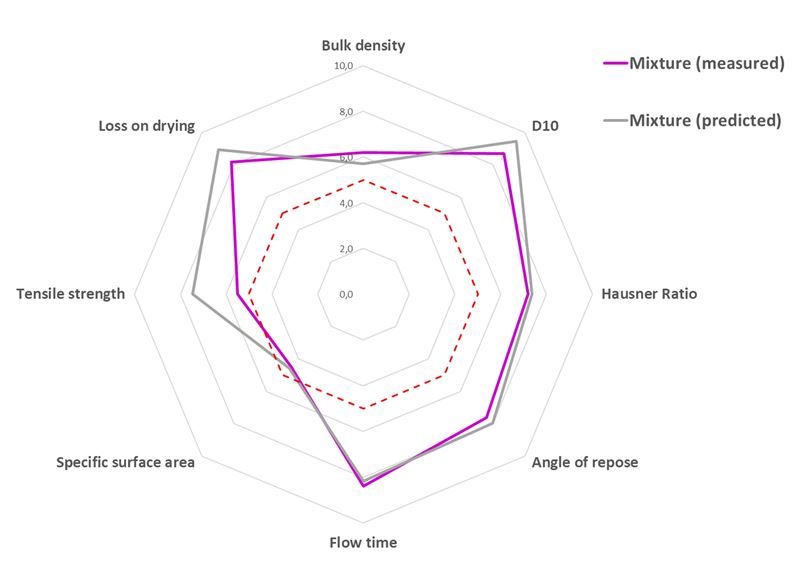
To assess prediction accuracy of the mixture behavior in DC, theoretical and experimental values are plotted on the same graph (figure 3).
It clearly appears on figure 3 that the performances of the studied ternary mixture (API/mannitol/MCC) are well predicted by the mode. The mean scores are almost identical (7.1/10 predicted vs 6.8/10 measured. This difference is mainly explained by a slight overestimation of the tensile strength.
Figure 3. Comparison of predicted and measured parameters for the powder mixture presented in figure 2. Measured mean score for mixture = 6.8/10.
Conclusion
The computational physics criteria presented here enable better prediction of powder mixture behavior in direct compression. It is based on the SeDeM system with improvement on several aspects. It first introduces specific surface area (associated with compactibility) and homogeneity index of powder mixtures. It does not take into consideration tap density (not connected to tabletability) and selected Hausner ratio over Carr index due to their opposite contribution to the overall evaluation. Finally, realistic ranges of acceptability for studied parameters were defined to better represent experimental values.
References
1. Pérez, Pilar, Josep M. Suñé-Negre, Montserrat Miñarro, Manel Roig, Roser Fuster, Encarna García-Montoya, Carmen Hernández, Ramón Ruhí, and Josep R. Ticó. “A new expert system (SeDeM Diagram) for control batch powder formulation and preformulation drug products.” European journal of pharmaceutics and biopharmaceutics 64, no. 3 (2006): 351-359.
https://doi.org/10.1016/j.ejpb.2006.06.008
2. Suñé-Negre, Josep M., Pilar Pérez-Lozano, Montserrat Miñarro, Manel Roig, Roser Fuster, Carmen Hernández, Ramon Ruhí, Encarna García-Montoya, and Josep R. Ticó. “Application of the SeDeM Diagram and a new mathematical equation in the design of direct compression tablet formulation.” European journal of pharmaceutics and biopharmaceutics 69, no. 3 (2008): 1029-1039.
https://doi.org/10.1016/j.ejpb.2008.01.020
3. Leane M., Pitt K., Reynolds G. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm Dev Technol. 2015, 20 (1):12-21.
https://doi.org/10.3109/10837450.2014.954728
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.