NEOSORB® P 650 C - SORBITOL
NEOSORB®
®Registered Trademark(s) of Roquette Frères
- Crystalline
Particle size mean diameter - 650 µm approx, Available as non-GMO
Applications
- 1. Solid Forms - Tablets
- Chewable Tablets
- Swallowable Tablets
- Effervescent Tablets
- 2. Solid Forms - Capsules
- Hard Capsules Fill
- 5. Other Solid Forms
- Losenges
- Medicated Confectionaries
- Stick Packs and sachets
- 3. Films and coatings
- Film Coating
- 4. Liquid Forms
- Creams | Pastes & Semi-Solids
Functional properties
- Formulations Aids
- Fillers and Binders for Direct Compression
- Fillers and Binders for Roller Compaction
- Humectants
- Sensory Enhancers
- Sweetening agent
- Stabilizers and Adjusters
- Plasticizers
Physical and chemical properties
- Health & Nutritional Benefits
- Sugar-free
- Gluten-free
- Non-cariogenic
- General Properties
- Label-friendly
- Multicompendial
Documents
Product Specification Sheet
Name
Region
Size
Download
NEOSORB® P650 C
490,85 Ko
Region :
490,85 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
NEOSORB® P650 C
Europe, BE
EN
519,28 Ko
Region : Europe, BE
EN
519,28 Ko
NEOSORB® P650 C
Americas, CA
EN
279,86 Ko
Region : Americas, CA
EN
279,86 Ko
NEOSORB® P650 C
Europe, CH
EN
519,43 Ko
Region : Europe, CH
EN
519,43 Ko
NEOSORB® P650 C
Asia, CN
EN
326,41 Ko
Region : Asia, CN
EN
326,41 Ko
NEOSORB® P650 C
Europe, DE
EN
521,99 Ko
Region : Europe, DE
EN
521,99 Ko
NEOSORB® P650 C
Europe, FI
EN
519,29 Ko
Region : Europe, FI
EN
519,29 Ko
NEOSORB® P650 C
Europe, FR
EN
519,34 Ko
Region : Europe, FR
EN
519,34 Ko
NEOSORB® P650 C
Europe, AT
DE
531,66 Ko
Region : Europe, AT
DE
531,66 Ko
NEOSORB® P650 C
Europe, BE
DE
531,53 Ko
Region : Europe, BE
DE
531,53 Ko
NEOSORB® P650 C
Europe, BE
FR
632,95 Ko
Region : Europe, BE
FR
632,95 Ko
NEOSORB® P650 C
Europe, BE
NL
527,54 Ko
Region : Europe, BE
NL
527,54 Ko
NEOSORB® P650 C
Europe, BG
BG
680,10 Ko
Region : Europe, BG
BG
680,10 Ko
NEOSORB® P650 C
Americas, BR
PT
537,78 Ko
Region : Americas, BR
PT
537,78 Ko
NEOSORB® P650 C
Americas, CA
FR
395,06 Ko
Region : Americas, CA
FR
395,06 Ko
NEOSORB® P650 C
Europe, CH
DE
531,77 Ko
Region : Europe, CH
DE
531,77 Ko
NEOSORB® P650 C
Europe, CH
FR
633,39 Ko
Region : Europe, CH
FR
633,39 Ko
NEOSORB® P650 C
Europe, CH
IT
621,48 Ko
Region : Europe, CH
IT
621,48 Ko
NEOSORB® P650 C
Asia, CN
ZH
645,36 Ko
Region : Asia, CN
ZH
645,36 Ko
NEOSORB® P650 C
Europe, CZ
CS
653,08 Ko
Region : Europe, CZ
CS
653,08 Ko
NEOSORB® P650 C
Europe, DE
DE
536,27 Ko
Region : Europe, DE
DE
536,27 Ko
NEOSORB® P650 C
Europe, EE
ET
625,69 Ko
Region : Europe, EE
ET
625,69 Ko
NEOSORB® P650 C
Europe, DK
DA
528,89 Ko
Region : Europe, DK
DA
528,89 Ko
NEOSORB® P650 C
Europe, ES
ES
534,21 Ko
Region : Europe, ES
ES
534,21 Ko
NEOSORB® P650 C
Europe, FI
FI
535,85 Ko
Region : Europe, FI
FI
535,85 Ko
NEOSORB® P650 C
Europe, FR
FR
633,16 Ko
Region : Europe, FR
FR
633,16 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
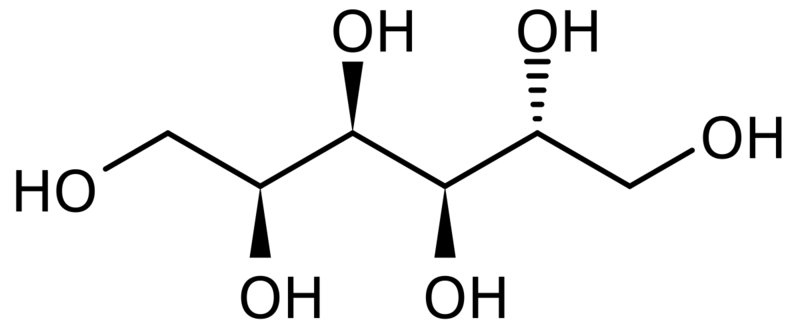
| Synonyms | D-Glucitol |
|---|---|
| CAS number | 50-70-4 |
| Physical form or apperance | White or almost white crystalline powder |
| Application | NEOSORB® P 650 C sorbitol is a filler and filler-binder excipient with excellent tableting properties. It is a non-cariogenic and non-acidogenic sugar-free sweetener that is suitable for chewable, suckable or effervescent tablets. |
| Teste/Odor | Slightly sweet (sweetening power about 50-60% that of sucrose) with cooling effect due to high dissolution enthalpy in water (111 J/g) |
| Morphology |
|
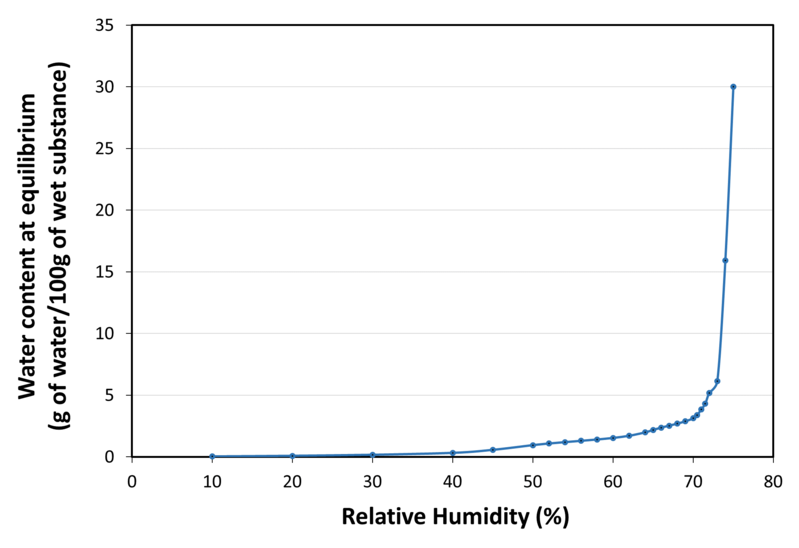
| Water sorption isotherm at 20°C |
|
| Chemical Structure |
|
| Average molecular weight | 182.2 g/mol |
| Nominal Water content (LOD) | 0.20 |
| Maximal Water content (LOD) | 1.50 |
| Solubility | Very soluble in water (235 g/100 ml of water at 25°C), practically insoluble in ethanol (96%) |
| Minimum melting temperature | 95 °C |
| Maximum melting temperature | 98 °C |
| Average mean particle diameter | 650 |
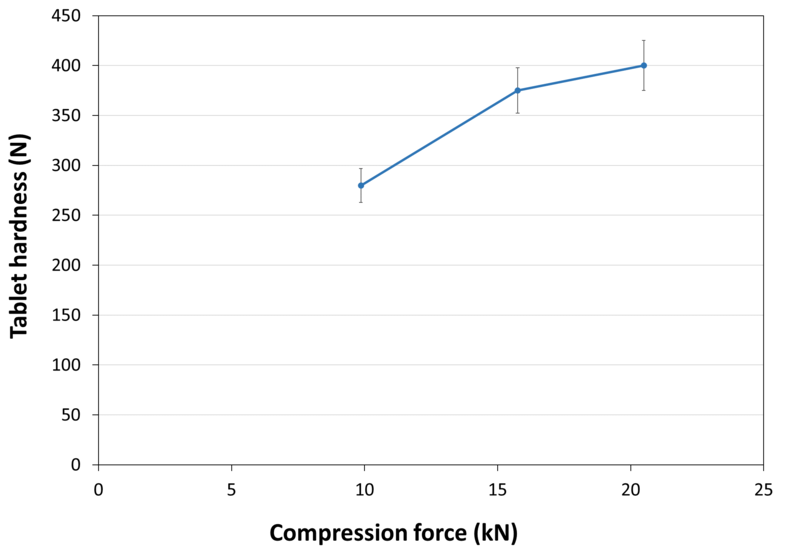
| Tablet Hardness |
|
| Experimental Conditions for Compression Behavior_Tablet Press | STYLCAM 200R |
| Experimental Conditions for Compression Behavior_Production Speed | 10 tabs/min (linear punch velocity: 38 mm/s ; simulated rotary press speed: 60000 tablets/hour) |
| Experimental Conditions for Compression Behavior_Tooling | Diameter 10 mm R9 concave |
| Experimental Conditions for Compression Behavior_Formuls | 99.3% NEOSORB® P 650 C / 0.7% magnesium stearate |
| Experimental Conditions for Compression Behavior_Tablet Mass | 400 mg |
| Powder Flowability (according to Ph.Eur. 2.9.16, 10mm outflow opening) | 11 |
| Bulk Density (g/ml) | 0.67 |
| Tapped Density (g/ml) | 0.69 |
| True Density (g/ml) | 1.51 |
| Specific Surface Area (m²/g) | 2.00 |
| Angle of Repose (°) | 30 |