KLEPTOSE® HPB PARENTERAL GRADE - HYDROXYPROPYLBETADEX
KLEPTOSE®
®Registered Trademark(s) of Roquette Frères
- Molecular encapsulation
- Improve injectable API solubility and/or stability
Medium molecular substitution (MS) = 0.58 - 0.68
Applications
- Injectable
- Rx Injectable
- Specialty APIs
Functional properties
- APIs
- Encapsulating agent
- Excipients
- Solubilizer
- Stabilizer
Physical and chemical properties
- CEP & DMF available
Documents
Product Specification Sheet
Name
Region
Size
Download
KLEPTOSE® HPB PARENTERAL GRADE
479,98 Ko
Region :
479,98 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
KLEPTOSE® HPB PARENTERAL GRADE
Oceania, AU
EN
265,62 Ko
Region : Oceania, AU
EN
265,62 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, BE
EN
511,81 Ko
Region : Europe, BE
EN
511,81 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Americas, BR
EN
511,51 Ko
Region : Americas, BR
EN
511,51 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Americas, BR
EN
528,87 Ko
Region : Americas, BR
EN
528,87 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Americas, CA
EN
277,45 Ko
Region : Americas, CA
EN
277,45 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, CH
EN
512,21 Ko
Region : Europe, CH
EN
512,21 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Asia, CN
EN
319,61 Ko
Region : Asia, CN
EN
319,61 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, DE
EN
514,94 Ko
Region : Europe, DE
EN
514,94 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, FI
EN
511,86 Ko
Region : Europe, FI
EN
511,86 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, FR
EN
512,25 Ko
Region : Europe, FR
EN
512,25 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, AT
DE
526,06 Ko
Region : Europe, AT
DE
526,06 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, BE
DE
525,88 Ko
Region : Europe, BE
DE
525,88 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, BE
FR
624,14 Ko
Region : Europe, BE
FR
624,14 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, BE
NL
519,92 Ko
Region : Europe, BE
NL
519,92 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Americas, CA
FR
390,11 Ko
Region : Americas, CA
FR
390,11 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, CH
DE
524,03 Ko
Region : Europe, CH
DE
524,03 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, CH
FR
626,55 Ko
Region : Europe, CH
FR
626,55 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, CH
IT
613,61 Ko
Region : Europe, CH
IT
613,61 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Asia, CN
ZH
633,49 Ko
Region : Asia, CN
ZH
633,49 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, CZ
CS
642,17 Ko
Region : Europe, CZ
CS
642,17 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, DE
DE
528,82 Ko
Region : Europe, DE
DE
528,82 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, DK
DA
521,41 Ko
Region : Europe, DK
DA
521,41 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, ES
ES
526,91 Ko
Region : Europe, ES
ES
526,91 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, FI
FI
528,28 Ko
Region : Europe, FI
FI
528,28 Ko
KLEPTOSE® HPB PARENTERAL GRADE
Europe, FR
FR
624,52 Ko
Region : Europe, FR
FR
624,52 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Synonyms | HYDROXYPROPYLBETADEX |
|---|---|
| CAS number | 128446-35-5 |
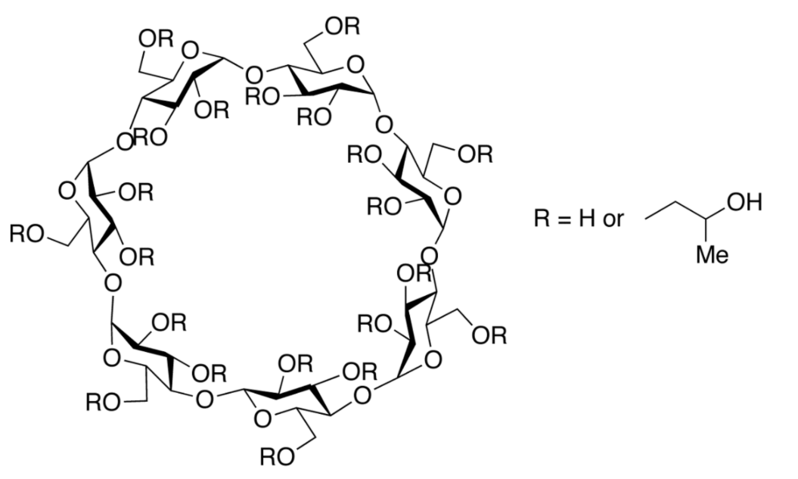
| Physical form or apperance | White or almost white amorphous powder. The statistical hydroxypropylation of beta-cyclodextrin creates a multitude of molecules, differing by the position of the hydroxypropyl groups. Consequently, the product is not able to crystallize and remains in the amorphous state. |
| Application | KLEPTOSE® HPB parenteral hydroxypropyl β-cyclodextrin. It is a pyrogen-free solution that improves drug solubility and stability against light or oxidation by molecular encapsulation. It also reduces irritation at injection site. |
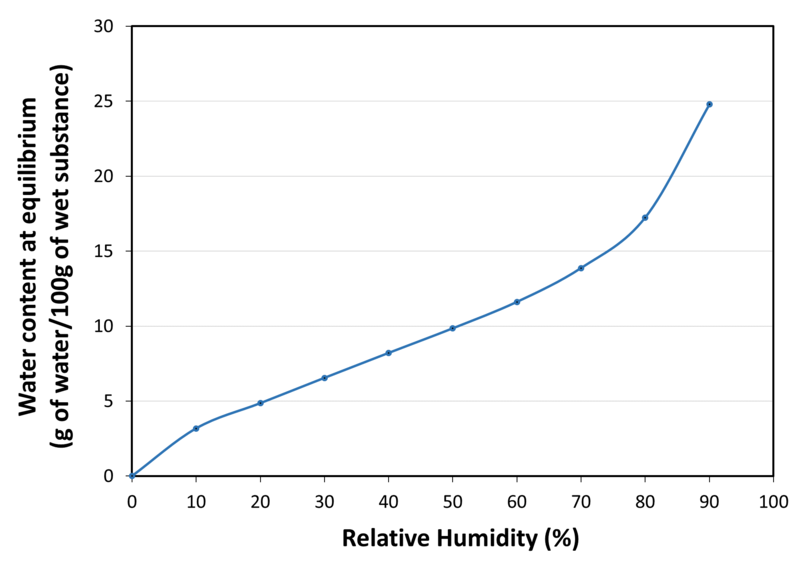
| Water sorption isotherm at 20°C |
|
| Chemical Structure |
|
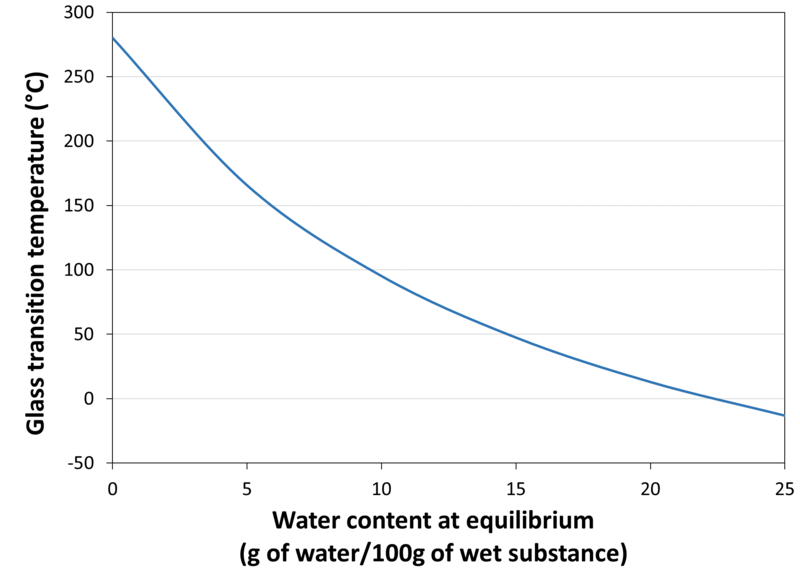
| Glass Transition Temperature vs. Water Content |
|
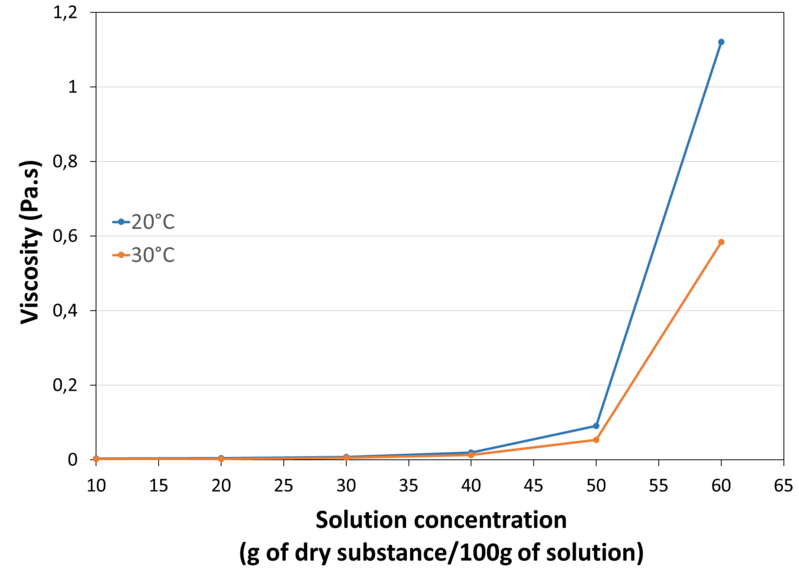
| Viscosity in water at 2°C |
|
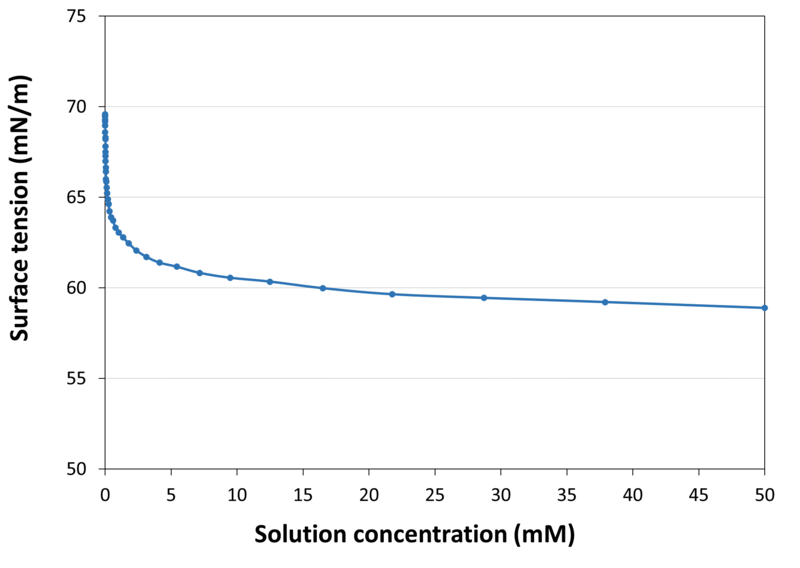
| Surface Tension |
|
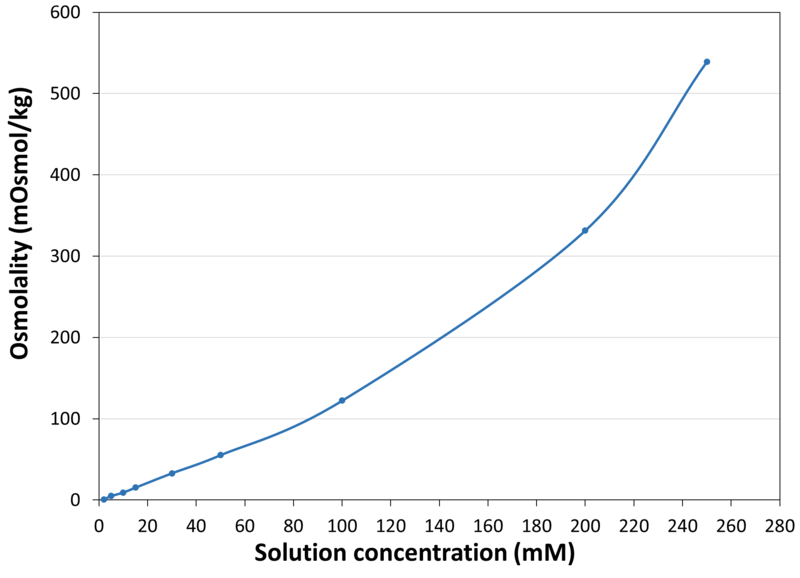
| Osmolality |
|
| Average molecular weight | 1387 g/mol |
| Nominal Water content (LOD) | 4.00 |
| Maximal Water content (LOD) | 10.00 |
| Nominal molar substitution | 0.62 |
| Minimum Molar substitution range | 0.58 |
| Maximum Molar substitution range | 0.68 |
| Solubility | Highly soluble in water. At high concentration, the wetting and dissolution time of the powder is delayed due to its low density and increased viscosity Soluble in ethanol. Freely soluble in propylen glycol. |
| Glass Transition Temperature of the Cryo-Concentrated Phase | -10.30 |