LYCASIN® 85/55 - MALTITOL SYRUP
LYCASIN®
®Registered Trademark(s) of Roquette Frères
- Concentrated solution enabling the addition of more water in the formulation
- High stability of medicine syrup
- Pleasant taste and sweet solution
- Non cariogenic
Content dry basis: 50-55%
Applications
- 5. Other Solid Forms
- Losenges
- Medicated Confectionaries
- 4. Liquid Forms
- Syrups | Suspensions and Liquids
Functional properties
- Formulations Aids
- Humectants
- Vehicles
- Sensory Enhancers
- Sweetening agent
Physical and chemical properties
- Health & Nutritional Benefits
- Sugar-free
- Low glycemic index
- Low insulin index
- General Properties
- Multicompendial
Documents
Product Specification Sheet
Name
Region
Size
Download
LYCASIN® 85/55
474,95 Ko
Region :
474,95 Ko
LYCASIN® 85/55
481,83 Ko
Region :
481,83 Ko
Safety Data Sheet
Name
Region
Language
Size
Download
LYCASIN® 85/55
Europe, BE
EN
407,23 Ko
Region : Europe, BE
EN
407,23 Ko
LYCASIN® 85/55
Europe, CH
EN
407,57 Ko
Region : Europe, CH
EN
407,57 Ko
LYCASIN® 85/55
Asia, CN
EN
211,24 Ko
Region : Asia, CN
EN
211,24 Ko
LYCASIN® 85/55
Europe, DE
EN
408,23 Ko
Region : Europe, DE
EN
408,23 Ko
LYCASIN® 85/55
Europe, ES
EN
268,61 Ko
Region : Europe, ES
EN
268,61 Ko
LYCASIN® 85/55
Europe, FI
EN
407,28 Ko
Region : Europe, FI
EN
407,28 Ko
LYCASIN® 85/55
Europe, FR
EN
407,30 Ko
Region : Europe, FR
EN
407,30 Ko
LYCASIN® 85/55
Europe, GB
EN
269,45 Ko
Region : Europe, GB
EN
269,45 Ko
LYCASIN® 85/55
Americas, Asia, Oceania, Africa
EN
156,29 Ko
Region : Americas, Asia, Oceania, Africa
EN
156,29 Ko
LYCASIN® 85/55
Europe, AT
DE
419,47 Ko
Region : Europe, AT
DE
419,47 Ko
LYCASIN® 85/55
Europe, BE
DE
419,42 Ko
Region : Europe, BE
DE
419,42 Ko
LYCASIN® 85/55
Europe, BE
FR
625,39 Ko
Region : Europe, BE
FR
625,39 Ko
LYCASIN® 85/55
Europe, BE
NL
520,83 Ko
Region : Europe, BE
NL
520,83 Ko
LYCASIN® 85/55
Americas, BR
PT
414,52 Ko
Region : Americas, BR
PT
414,52 Ko
LYCASIN® 85/55
Europe, CH
DE
419,71 Ko
Region : Europe, CH
DE
419,71 Ko
LYCASIN® 85/55
Europe, CH
FR
625,59 Ko
Region : Europe, CH
FR
625,59 Ko
LYCASIN® 85/55
Europe, CH
IT
611,65 Ko
Region : Europe, CH
IT
611,65 Ko
LYCASIN® 85/55
Asia, CN
ZH
497,14 Ko
Region : Asia, CN
ZH
497,14 Ko
LYCASIN® 85/55
Europe, DE
DE
420,49 Ko
Region : Europe, DE
DE
420,49 Ko
LYCASIN® 85/55
Europe, DK
DA
417,66 Ko
Region : Europe, DK
DA
417,66 Ko
LYCASIN® 85/55
Europe, ES
ES
421,41 Ko
Region : Europe, ES
ES
421,41 Ko
LYCASIN® 85/55
Europe, FI
FI
424,28 Ko
Region : Europe, FI
FI
424,28 Ko
LYCASIN® 85/55
Europe, FR
FR
625,38 Ko
Region : Europe, FR
FR
625,38 Ko
LYCASIN® 85/55
Americas, Asia, Oceania, Africa
ES
167,03 Ko
Region : Americas, Asia, Oceania, Africa
ES
167,03 Ko
LYCASIN® 85/55
Americas, Asia, Oceania, Africa
PT
169,41 Ko
Region : Americas, Asia, Oceania, Africa
PT
169,41 Ko
Get in touch with our Technical Experts
Please feel free to contact our technical experts for support during the development process.
Technical data
| Synonyms | Hydrogenated Starch Hydrolysate |
|---|---|
| Composition | Mixture of mainly D-maltitol (maltitol content on dry basis: 50-55%) with D-sorbitol and hydrogenated oligo - and polysaccharides |
| CAS number | D-Maltitol 585-88-6; Sorbitol 50-70-4 |
| Physical form or apperance | Clear, colorless, syrupy liquid |
| Application | LYCASIN® 85/55 liquid maltitol is used as a vehicle and a humectant for syrups and suspensions, and as a bulk sweetener in lozenges and medicated confectionary. It is a highly concentrated maltitol solution with less water. It thus enables to add more water in the formulation to dissolve other components like API. LYCASIN® 85/55 contains maltitol, sorbitol and other hydrogenated oligomers, and has a high chemical and microbial stability. It can be used as a substitute for sucrose and meets organoleptic (natural sweetness) and health requirements (it is sugar-free, low-calorie, does not induce dental caries, and may be advised for diabetics). |
| Teste/Odor | Pleasant taste and sweet solution |
| Chemical Structure |
|
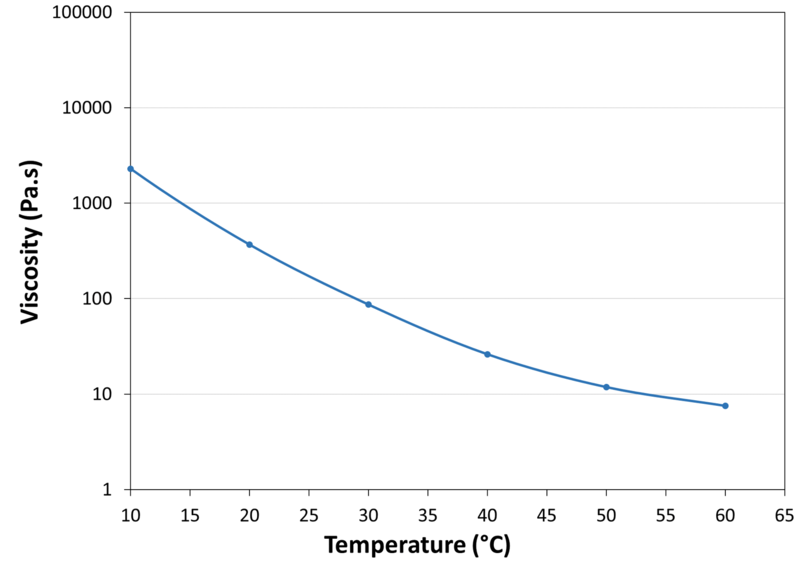
| Viscosity vs. Temperature |
|
| Average molecular weight | D-Maltitol 344 g/mol; Sorbitol 182.2 g/mol |
| Nominal Water content (LOD) | 15.00 |
| Maximal Water content (LOD) | 32.00 |
| Solubility | Miscible with water and with glycerol |
| Water Activity | 0.61 |