Why Oral Disintegrating Tablets?
Published February 03, 2026
Carmen Popescu, PhD, Technical Developer, Roquette, provides an overview of the Orally Disintegrating Tablet (ODT) landscape, describing how these dosage forms can be used to improve and differentiate drug products, different manufacturing methods and how this class of oral delivery system might be applied in the future.
Original publication: Popescu C. Why Oral Disintegrating Tablets? Drug Delivery Magazine, Issue 69 (Jul 2016), pp 35-38. ©2016 Frederick Furness Publishing Ltd.
Dr Carmen Popescu obtained her BSc in Physics and PhD in Biophysics from the University of Bucharest, Romania. She is a Technical Developer, former Senior Project Coordinator at Roquette America Inc, and Adjunct Associate Professor with University of Illinois at Chicago, Roosevelt University and University of Tennessee. She has published more than 120 research papers, book chapters and presentations on classic and new drug delivery dosage forms for small and large molecules. Additionally, Dr. Popescu is a reviewer for the International Journal of Pharmaceutics, Journal of Pharmaceutical Sciences, European Journal of Pharmaceutics and Biopharmaceutics, and Journal of Pharma & Pharmaceutical Science. Dr. Popescu is also an active member of American Association of Pharmaceutical Scientists and the Controlled Release Society.
Introduction
Oral disintegrating tablets (ODTs) are patient-centric drug delivery systems (for example, for pediatrics, geriatrics, and psychiatric patients with dysphagia) designed to increase patient compliance. ODTs are preferred to classic dosage forms (swallowable / chewable / suckable tablets) due to ease of administration (portability, “on the go”) without water, pleasant taste and mouthfeel – more of “a treat” than a treatment. Reduced first-pass metabolism, faster onset of action, better absorption and, in turn, improved bioavailability are their very appealing benefits. Manufacturers’ attraction for these dosage forms resides in improved lifecycle management, market differentiation, innovation and brand creation. Moreover, in recent years, we can see their remarkable expansion from Rx to OTC, nutraceuticals (vitamins, minerals, etc.) and biologics. In response to the increased popularity of ODTs on the market, the excipients industry created ready-to-use platforms in order to ease the formulation process.
Recently, the U.S. FDA approved SPRITAM (levetiracetem) ODT tablets produced by 3D printing. This is a significant step towards personalized drug delivery.
Dr Carmen Popescu
What are the main requirements for an ODT?
As per the US and EU pharmacopoeia guidelines, an ODT usually weighs 500 mg (EP, USP) or less, disintegrate in 2 mL available saliva in less than 30 seconds (USP) or 180 seconds (EP) and the friability is to be ≤1% (EU, USP).1, 2, 3.
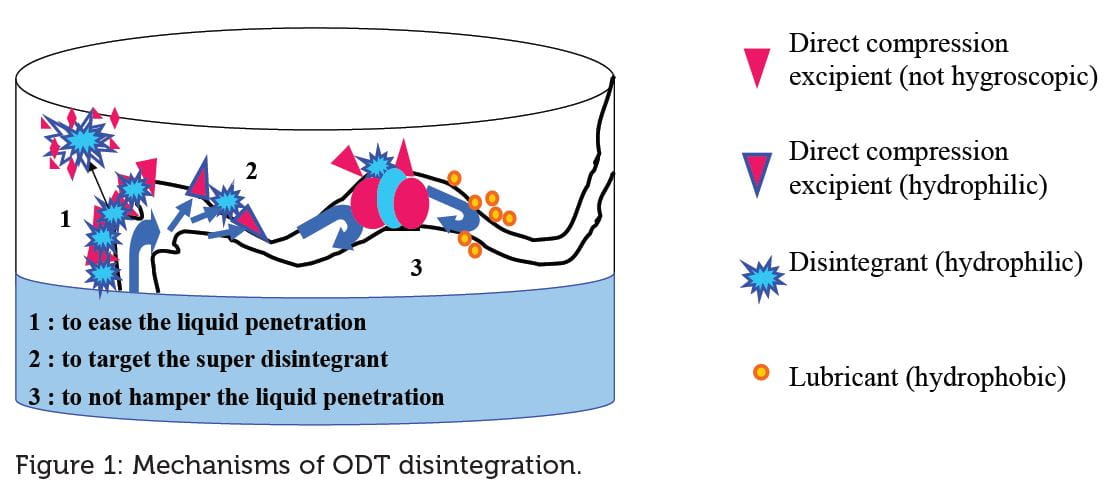
In order to satisfy these requirements, the filler has to create a porous matrix in which the 2 mL saliva will be fast-channelled to the super disintegrant in order to break down within 30 seconds (Figure 1). Mannitol is the chosen filler (but there are other candidates like dextrose, lactose, starch, etc.) due to it being water-soluble but not hygroscopic (reduce interaction with the water penetration through the matrix pores) and protects actives stability.
What processes are available to manufacture ODTs?
Freeze-drying, spray-drying, direct compression, molding sublimation, mass extrusion, and cotton candy are the commonly used methods in the industry. Amongst these, direct compression is the most cost-effective and easy-to-handle on standard equipment, resulting in low-friability tablets.
In recent years, the excipients' industry has developed a number of ready-to-use ODT platforms by co-processing the filler, usually mannitol, with a superdisintegrant. The platforms include:
- F-MELT® (Fuji Chemical Industries, Tokyo, Japan)
- Ludiflash® (BASF, Ludwigshafen, Germany)
- Parteck® ODT (Merck Millipore, Billerica, MA, US)
- PEARLITOL® Flash Mannitol-Starch Compound (Roquette Pharma, Lestrem, France)
- Pharmaburst® (SPI Pharma, Wilmington, DE, US)
- PROSOLV® ODT (JRS Pharma, Rosenberg, Germany)
The main challenge in ODT formulation is the screening of excipients in order to find the right balance between disintegration time, friability, API stability and mouthfeel. These aspects are explored in greater detail in the case studies that follow.
Case study 1: Disintegration-time optimization of ready-to-use platforms
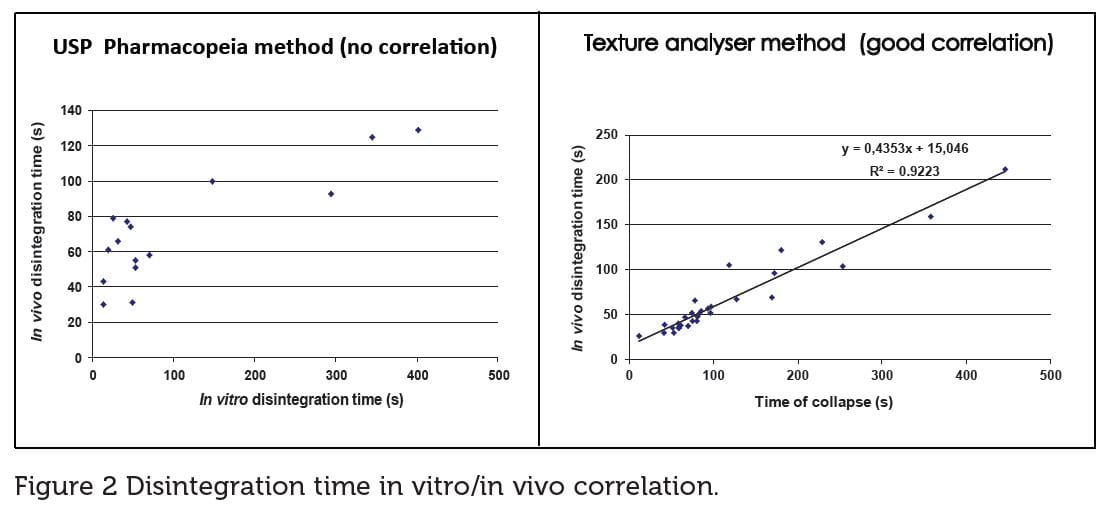
Disintegration time can be evaluated in vitro as per the USP/EU Pharmacopoeias’ methods or any method using Texture Analyzer and their correlation with in vivo evaluation by a taste panel (Figure 2).
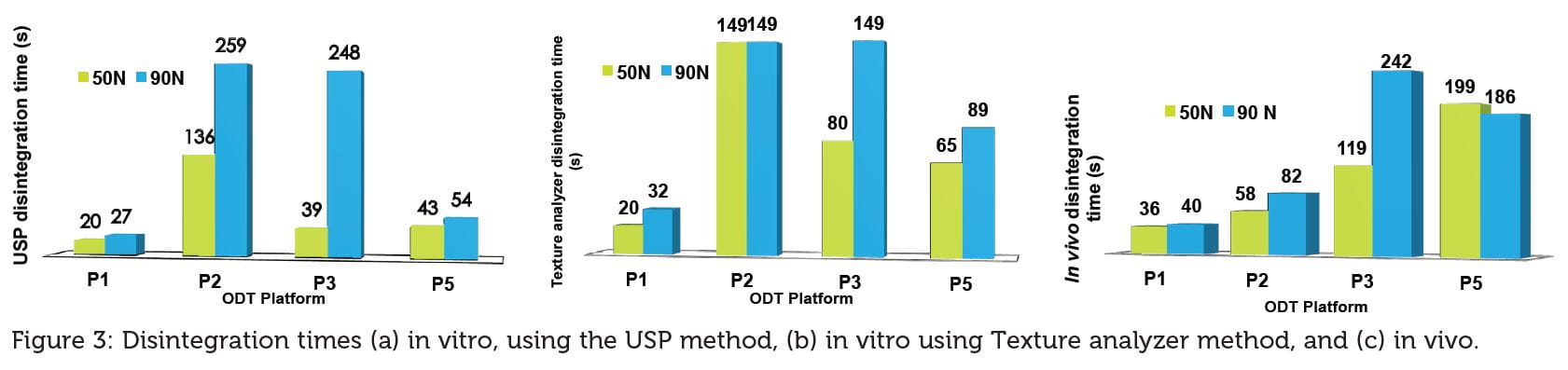
300 mg ODT placebos were made using ready-to-use ODT platforms at two hardness values (50 N and 90 N), and their disintegration times were evaluated in vitro (Figure 3a & b) and in vivo (Figure 3c).
ODT platforms composition filler: disintegrant is as follows: P1 (Mannitol: Starch); P2 (Mannitol: Crospovidone, PVA, PVP, SLS); P3 (Mannitol: Crospovidone, MCC, SiO2, Fructose) and P4 (Mannitol: Croscarmellose).
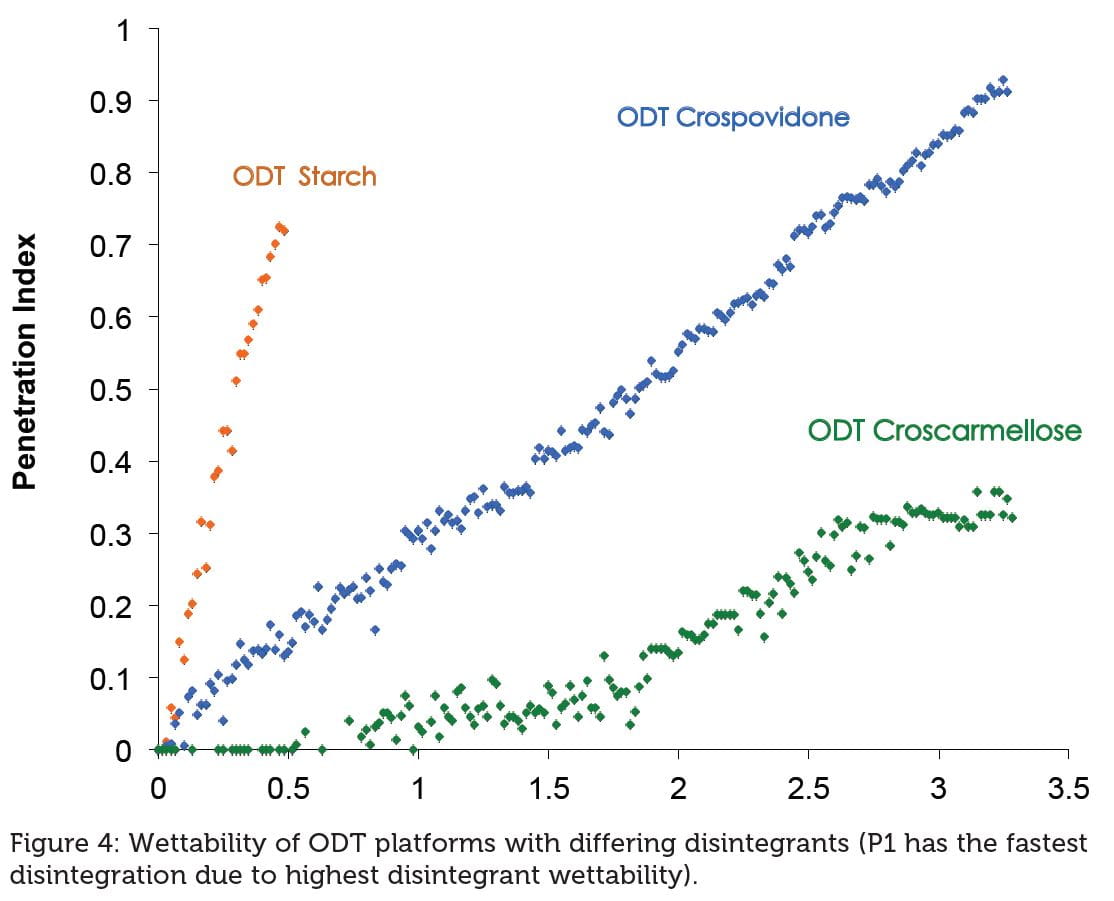
Tablet hardness has no effect on the disintegration time in vitro (both methods Figure 3a and Figure 3b) or in vivo (Figure 3c) for P1 while for the other platforms there is noticeable variation as a function of hardness. The reason for P1’s short disintegration time resides in the water access (through porous matrix) to the disintegrant due to its superior wettability compared with the other platforms (Figure 4).
Case study 2: Impact of ODT platform composition on mouthfeel
Twenty-four trained panelists were asked to put an ODT placebo between tongue and palate applying a slight pressure and then their opinion about the mouthfeel was recorded. Mouthfeel is critical in patient acceptance of an ODT (due to its residence time in the buccal area) and is very much linked to attributes such as smooth, creamy, sweet, etc. Unfortunately, for some ODT platforms, the synthetic origin of their components seems to affect their taste and texture negatively.
The taste panel evaluation of P1 to P4 commercially available ODT platforms were as follows:
P1: Sweet taste, creamy, smooth and fine texture and off notes (Medicinal)
P2: Creamy and sticky texture and off notes (Dry, Glue, Cardboard, Bitter)
P3: Not very sweet, takes too long time to melt and off notes (Cardboard, Bitter)
P4: Takes too long to melt, hard center and off notes (Cardboard, Chemical)
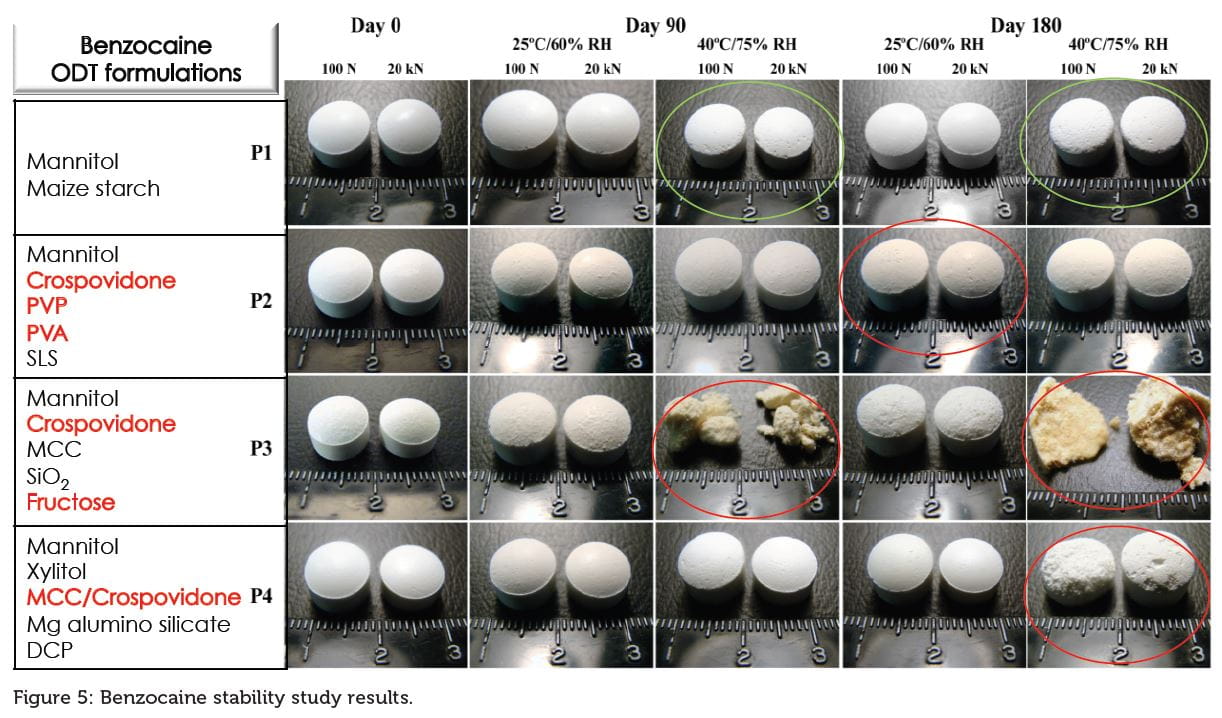
Case study 3: Impact of ODT platform composition on chemical and physical stability
ODTs were formulated with 6% benzocaine (as a model drug), 1.5% magnesium stearate, and 92.5% of the respective P1, P2, P3 ODT platforms (as described previously) and P4 (mannitol: xylitol, MCC, crospovidone, Mg alumino silicate, DCP). Each formulation was tableted at 500 mg weight using 10 mm diameter concave punches on a Korsch XP1 research tableting machine under two conditions.The tablets in the first set were produced at different compression force depending on platform compressibility to create tablets with an average hardness of 100 N. The tablets in the second set were made under a constant compression force of 20 kN, which resulted in tablets with varying hardness. Tablets were evaluated in accordance with US Pharmacopoeia methods for hardness, friability, and in vitro disintegration time. Tablets were placed under ICH stability conditions in humidity chambers at 25°C/60% RH or under accelerated conditions, 40°C/75% RH, for up to six months in open pans. Following storage under the various stability conditions, tablets were photographed and their diameter measured. Benzocaine was chosen as a model drug due to its propensity (H2N group) to degrade under certain circumstances (reducing sugars, formic acid, and formaldehyde) and its degradation (Brown Millard reaction, N-Formyl benzocaine, p-amino benzoic acid, amide degradation product) under stability conditions was evaluated by LCMS.
Physical stability was impacted by reducing sugar (fructose), superdisintegrants and MCC (see Figure 5), while chemical stability was impaired by reducing sugar (fructose) and reactive residues (peroxides, formic acid and formaldehyde) in crospovidone, PVP or PVA. P1 and P2 displayed a very good chemical and physical stability.4
ODT by 3D printing: a transition to personalized medicine
Recently, the US FDA approved SPRITAM (levetiracetem) ODT tablets produced by 3D printing. This is a significant step towards personalized drug delivery being tailored to the individual patients based on their predicted response or risk of disease. The treatment will be more cost-effective and accurate or, in other words, “therapy with the right drug at the right dose in the right patient”.5
Giving practicality to novelty
It is well known that more than 45% of new drug entities have solubility issues, and micronization / nanonization is one way to address this problem. However, reducing the particle size at the micro / nano scale, the drug is usually delivered only as an injectable. Normally it cannot be formulated as a conventional tablet because, during compression, particles will aggregate resulting in bigger particle size and solubility reduction. However, in the case of ODTs, due to low compression force applied, the particle size is not changed.
For the same reasons, ODTs are potentially very suitable for protein and peptide delivery, preserving their structure, with delivery in the buccal cavity increasing their bioavailability. As the next generation of drugs coming through pipelines are increasingly biopharmaceuticals, ODT represents a viable option for their oral delivery.
References
- US FDA, “Guidance for Industry.” 2008.
- US Pharmacopeia, 36, 2013.
- European Pharmacopeia, 8.2, 2014.
- Köllmer M, Popescu C, Manda P, Zhou L, Gemeinhart RA. “Stability of Benzocaine Formulated in Commercial Oral Disintegrating Tablet Platforms.” AAPS PharmSciTech, 2013, vol. 14(4), pp. 1333-40.
- Mancinelli L, Cronin M, Sadée W. “Pharmacogenomics. The Promise of Personalized Medicine.” AAPS PharmSciTech, 2000, vol. 2(1), E4.