Troubleshooting and FAQs for KLEPTOSE® β-cyclodextrins and hydroxypropyl-β-cyclodextrins
Frequently asked questions for KLEPTOSE® beta-cyclodextrins and hydroxypropyl-beta-cyclodextrins and their applications
-
Cyclodextrins are not sensitive to filtration or autoclaving processing conditions.
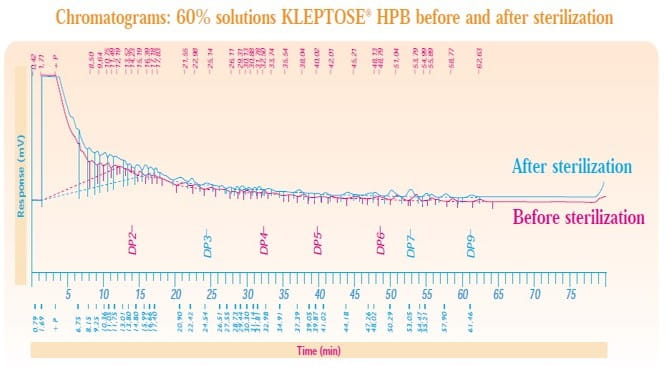
Aqueous HPBCD solutions of various concentration (from 10 % to 60 % of KLEPTOSE® HP and KLEPTOSE® HPB) were sterilized for 15 minutes at 121 °C. No degradation of hydroxypropyl-β-cyclodextrin solutions occurred during sterilization:
• The coloration of the solution (ICUMSA coloration) did not increase with sterilization.
• The reducing sugar content (Somogyi-Nelson method) did not increase with sterilization.
• The sugar spectra using High Performance Anion Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD) showed an identical profile for the solutions before and after sterilization. An example of the chromatogram for 60 % solution KLEPTOSE® HPB before and after sterilization is shown below:
-
The molar substitution of both the oral and parenteral grades of KLEPTOSE® HP are the same, and are both generally used in small molecule applications. The key difference of the parenteral and oral grades of KLEPTOSE® HP are listed in the table below.
-
Hydroxypropyl-ß-cyclodextrins are not surfactants. Unlike a surfactant, they do not have a critical micellar concentration (CMC) value. However, it has been reported that hydroxypropyl-ß-cyclodextrins exhibit some surface active properties, where it was observed that the surface tension generally decreases as the concentration of hydroxypropyl-ß-cyclodextrin increases.
-
Usually a combination of analytical techniques are used to understand the complexation between the guest molecule and cyclodextrin due to the inherent limitations and sensitivity of the respective techniques. Nuclear magnetic resonance (NMR) spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC), power X-ray diffraction are some of the common methods employed.
For example, DSC may be used to understand the complexation of a crystalline drug molecule with cyclodextrin. For a crystalline drug molecule, sharp melting point peaks may be observed on the thermograms. If there is a broad melting range and no specific melting peak corresponding to the drug molecule, it may suggest the formation of an inclusion complex.
-
In the presence of water, cyclodextrins (host molecule) can form inclusion complexes with many drugs (guest molecule) by taking up the molecule, or more frequently some lipophilic part of the molecule, into the central cavity by a steric and a thermodynamic interaction. Drug molecules in the complex are in rapid equilibrium with free molecules in the solution. No covalent bonds are formed or broken during the complex formation. Examples of forces involved in the complex formation are as follows:
• Release of enthalpy-rich water molecules from the cavity
• Electrostatic interactions
• Van der Waals’ interactions
• Hydrophobic interactions
• Hydrogen bonds
• Release of conformational strain and charge-transfer interactionsReference: Frömming KH, Szejtli J. Cyclodextrins in pharmacy. Springer Science & Business Media; 1993
-
Inclusion compounds with cyclodextrins can be prepared in various ways, such as spray-drying, freeze-drying, kneading, physical mixing, etc. The preparation method may be selected from some preliminary trials to determine the complexation efficiency for the given method. To prepare complexes in the solid form, the solvent needs to be removed in the final step of the process.
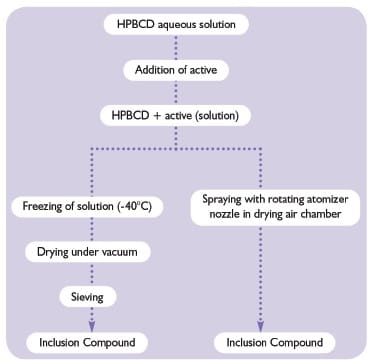
With the hydroxypropyl-β-cyclodextrins (HPBCD), the preparation of inclusion compounds or complexes in aqueous media is very simple. The general principle involves the solubilization of the predetermined amount of HPBCD (KLEPTOSE® HP, KLEPTOSE® HPB or KLEPTOSE® HPB-LB). An aqueous solution is obtained instantly. The active ingredient is added to this solution and mixed until a clear solution is formed. Ultimately, the complex can be freeze-dried or spray-dried.For more information!: https://www.roquette.com/innovation-hub/pharma/expert-opinion/kleptose-hpb-lb-parenteral-grade-a-new-multi-compendia-modified-bcd
-
β-Cyclodextrins (β-CDs) are cyclic oligosaccharides with a bucket-like structure having a hydrophobic internal cavity and a hydrophilic exterior. This unique structure allows for the formation of inclusion complexes, where lipophilic compounds are non-covalently bound within the cavity.
-
The molar substitution of both the parenteral and biopharma grades of KLEPTOSE® HP and KLEPTOSE® HPB are the same. Both are low endotoxins grades that are suitable for parenteral dosage forms. However, the key difference is that KLEPTOSE® HP Biopharma and KLEPTOSE® HPB Biopharma undergoes additional testing beyond the monograph, such as Beta Glucans, DNAse, protease, residual DNA, ICH Q3D elemental impurities, and pesticides levels.
-
Native cyclodextrins and cyclodextrin derivatives such as hydroxypropyl-β-cyclodextrin (HPβCD) are used in marketed pharmaceutical products. The safety profiles of native cyclodextrins are well established, and they are accepted as food additives and “generally regarded as safe” (GRAS). For HPβCD is listed in the US, EP and JP Pharmacopoeias. Data on children under 2 years old treated with oral solutions of itraconazole with up to 200 mg HPβCD/kg/day for 2 weeks were well tolerated and considered safe. Nevertheless, it is important to note that the approval of cyclodextrin and the acceptable concentration can vary between territories or regions.
Also, not all cyclodextrins are recommended for all routes of administration. For example, both α-CD and β-CD showed renal toxicity after parenteral administration and are thus generally not recommended for medicinal products given intravenously. The table below lists the use of cyclodextrins in pharmaceutical products according to the route of administration.With the wide application of HPβCD in various routes of administration, Roquette has developed a range of products that are suited for your needs, including that of biopharma and parenteral applications.
-
Roquette has developed a range of substituted hydroxypropyl-β-cyclodextrins with different degrees of substitution that are described by the molar substitution level. KLEPTOSE® HP has a higher degree of molar substitution (0.81-0.99) compared to KLEPTOSE® HPB (molar substitution range of 0.58-0.68).
Both KLEPTOSE® HP and HPB are available in oral, parenteral and biopharma grades that are suitable for taste masking, solubility improvement and stability improvement of active molecules and therapeutic proteins. -
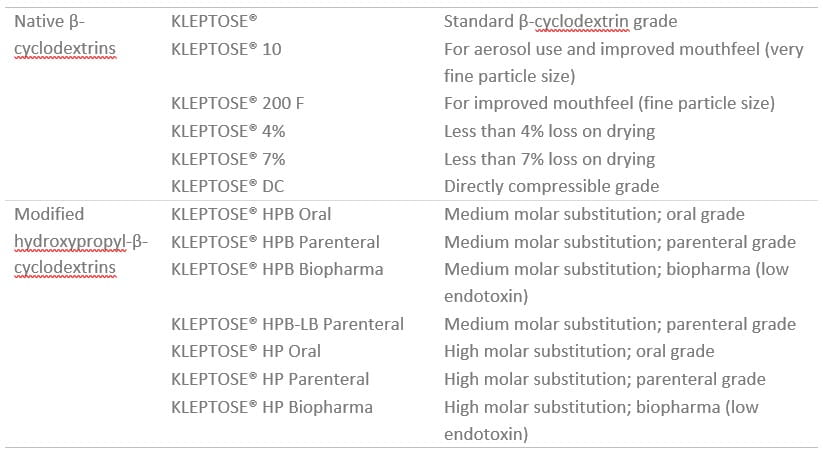
Roquette has a range of native and modified hydroxypropyl-β-cyclodextrins (HPBCD) for use in many dosage forms, from solid to liquid. The key attributes are listed for each product in the table below.
-
The use of cyclodextrins in small molecule drug formulations can be considered in some of the following cases:
1. When the active ingredient is poorly water soluble, which may then affect the bioavailability.
2. When the time required to reach the effective blood level of the orally administered drug is too long because of slow dissolution rate and/or incomplete absorption.
3. When there is a need to formulate an aqueous eye drop or injectable solution containing a poorly water soluble active ingredient.
4. When the active ingredient is physical or chemically unstable.
5. When there is poor acceptability of the drug due to the bad smell, bitter, astringent or irritating taste.
6. When some relief of side effects (e.g., throat, eye, skin or stomach irritation) is required.
7. When the active ingredient is available as a liquid, but the preferred pharmaceutical form is a stable tablet, powder, aqueous spray, etc.
Reference: Szejtli J. Cyclodextrins: applications. Encyclopedia of Supramolecular Chemistry. 2004;1:405-413. -
There are general preconditions to form a medicinally useful inclusion complex with the compound of interest. Firstly, it is important to understand the properties of the compound of interest.
In the case of small molecules, the following characteristics may be considered:
• Typically more than 5 atoms (C, P, S and N) to form the skeleton of the molecule
• Typically less than 5 condensed rings in molecule
• Solubility in water of less than 10 mg/ml
• Melting point temperature below 250 °C (otherwise, the cohesive forces between the molecules are too strong)
• Number of condensed rings: typically less than 5
• Molecular weight between 100 and 400 (with smaller molecules, the drug content of the complex is too low, and large molecules will not be able to fit into the cyclodextrin cavity)
• Electrostatic charges present on the molecule
For large molecules, it is expected that the molecular size would preclude a full complexation within the cyclodextrin cavity. However, the side chain in the macromolecules may contain suitable groups (e.g., aromatic amino acid in a polypeptide) that can interact with cyclodextrins in aqueous solutions and form a partial complex with cyclodextrins. It has been reported the stability of an aqueous solution of insulin, or other peptides, proteins, hormones and enzymes, have been significantly improved in the presence of an appropriate cyclodextrin.Taking the above into consideration, the next step would be to conduct laboratory trials to evaluate if the functional property (e.g., improved stability, solubility enhancement) has been attained with the cyclodextrin.
Reference: Szejtli J. Cyclodextrins: applications. Encyclopedia of Supramolecular Chemistry. 2004;1:405-413.