Tableting Study for Orodispersible Tablets ODTs
This joint tableting study by Laboratoire Pierre Fabre and Roquette highlights some of the ODT production benefits of PEARLITOL® Flash.
Situation
Orodispersible tablets or orally disintegrating tablets (ODTs) are dosage forms formulated in such a way as to improve a pharmaceutical product’s in vivo oral disintegration and dissolution rates. In order to achieve rapid disintegration rates, the tablet formula must provide a high porosity, low density and low hardness.1, 2
The most common preparation methods used to produce tablets are: molding, lyophilization, freeze-drying and direct compression.3-7 The basic approach of developing ODTs via direct compression involves the blending of a filler, superdisintegrant, a lubricant and an active pharmaceutical ingredient (API) and then compressing the mixture.8-11
Mannitol is commonly used as a diluent or bulk excipient in the formulation of ODTs. A new generation of coprocessed mannitol-based excipients has been developed by Roquette for the formulation of ODTs. For example, PEARLITOL® Flash is a combination of mannitol and starch. Mannitol and starch are compliant with international pharmacopoeias and the combined ingredient is specifically designed to disintegrate rapidly, providing a smooth texture without the addition of superdisintegrant.
Challenge
In the study presented here, the physical properties of PEARLITOL® Flash were evaluated through a series of tests that revealed the impact of different compression parameters (precompression and compression forces, tableting speed) on tablet properties while using two different types of conventional tablet presses.
The materials used in this study were PEARLITOL® Flash (a combination of mannitol and starch from Roquette) and magnesium stearate as a lubricant (purchased from Bärlocher). The powder properties, such as bulk and tapped density, angle of repose (using Hosokawa equipment), the flow rate through an orifice (using FloDex), the Hausner ratio and the Carr index, were all determined according to US Pharmacopoeia (USP) and European Pharmacopoeia (Ph. Eur.) methods in order to characterize the physical properties of PEARLITOL® Flash.
Particle size distribution (sieving), loss on drying (IR method, Mettler LP16) and sorption of water (Dynamic Vapour of Sorption 20°C, DVS-1, Surface Measurement Systems LTD) were also evaluated. The Scanning Electron Microscopy (SEM) was performed using a Quanta 200FEG.

Figure 1: Morphology of PEARLITOL® Flash (SEM, magnification 200)
Table 1: Formulas
|
Formula (% w/w) |
Korsch XP1 press |
Stylcam Simulator |
|---|---|---|
|
PEARLITOL® Flash coprocessed mannitol starch |
99.6 |
99.5 |
|
Magnesium stearate |
0.4 |
0.5 |
Results
Physical properties
The prepared tablets were evaluated for physical parameters, for example, thickness using a standard micrometer and hardness using Schleuniger 2E hardness tester. The disintegration time of the PEARLITOL® Flash tablets was also measured using the USP method.
The physical properties of PEARLITOL® Flash powder are shown in Table 2. It is a free-flowing powder consisting of white, colorless, agglomerated particles of 150 μm mean diameter (Figure 1). These data confirm that the material has flow properties well adapted to direct compression, even at high production rates. Due to its non-hygroscopic nature, mannitol is a versatile excipient used to formulate moisture sensitive drugs. This intrinsic property of mannitol is transferred to PEARLITOL® Flash despite the fact that it is combined with starch (Figure 2).
Table 2: Physical properties of PEARLITOL® Flash powder
|
Bulk density (g/mL) |
0.527 |
|---|---|
|
Tapped density (g/mL) |
0.631 |
|
Flowability (g/sec) |
20 |
|
Flodex (mm) |
4 |
|
Angle of repose (°) |
40 |
|
Hausner ratio |
1.19 |
|
Carr index (%) |
16.5 |
|
Loss on Drying (%) |
1.1 |
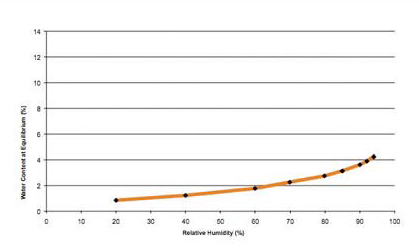
Figure 2: Dynamic vapor sorption isotherm of PEARLITOL® Flash at 20 °C
The tests carried out using varying compression settings with the Korsch XP1 provided an insight into the influence of compression force on ODT tablet performance.
PEARLITOL® Flash was compacted at forces of 10, 15, 20 and 25 kN. At 5 kN, the cohesion of the tablets was too low. Then the hardness of the tablets increased with the increased compression force. The 15 kN compression force was sufficient to obtain tablets with acceptable hardness, friability and disintegration times (see Figures 3 and 4, and Table 3).
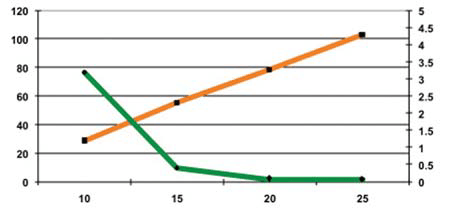
Figure 3: Hardness and friability as a function of compression force
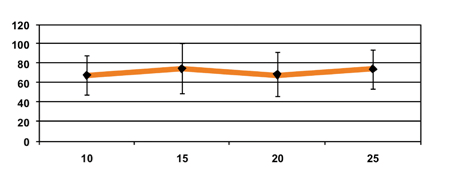
Figure 4: Disintegration time as a function of compression force
Surprisingly, the disintegration time was not significantly affected by the increase of the compression force and the tablet hardness. Generally, an increase in the compression force has a direct effect of lowering the disintegration time as it increases the hardness of the tablet and decreases its porosity. In ODTs, the absence of this interdependence between the disintegration time and the compression force has not previously been described.
Tableting speeds
To show the influence of tableting speed on ODT performance, tablets were produced at 20, 40 and 60 tablets/min. No significant impact on the tablet properties was observed while increasing tableting speed (20-60 tablets/min) (Table 4 and Figure 5). Tablet mass uniformity met the requirements of the Ph. Eur (m ± 5%). The hardness remained quite constant, while the disintegration time was lower but not statistically different for the higher tableting speed. For all tableting speeds, the increase in speed resulted in an increase of ejection force, but it still remained very low (under 230 N). The low values for the ejection forces (Tables 3 and 4) confirm that 0.4% of magnesium stearate is enough to lubricate PEARLITOL® Flash.
Table 3: Physical properties of PEARLITOL® Flash tablets (mean ± SD)
|
Compression Force (kN) |
10 |
15 |
20 |
25 |
|---|---|---|---|---|
|
Weight (mg) |
503.2 ± 1.30 |
505.3 ± 0.46 |
504.2 ± 1.56 |
505.6 ± 0.30 |
|
Ejection force (N) |
126 |
164 |
188 |
217 |
|
Hardness (N) |
28 ± 1 |
55 ± 1 |
79 ± 1 |
103 ± 2 |
|
Friability (%) |
3.22 |
0.42 |
0.14 |
0.07 |
|
Density (g/mL ) |
1.163 |
1.212 |
1.279 |
1.318 |
|
Porosity (%) |
22.3 |
19 |
14.5 |
11.9 |
|
Disintegration time (s) |
68 ± 20 |
75 ± 26 |
69 ± 23 |
74 ± 20 |
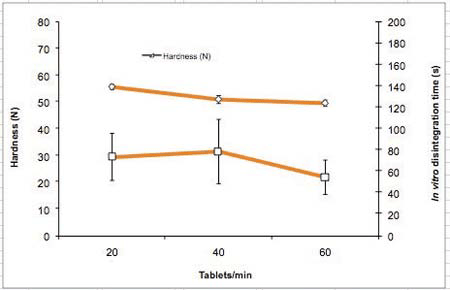
Figure 5: Hardness and in vitro disintegration time as a function of tableting speed (compression force 15 kN)
Table 4 shows the tableting speed study which includes the compression and tablet data for a 15 kN compression force (mean ± SD). Figure 5 also shows the hardness and in vitro disintegration time as a function of tableting speed (compression force 15 kN).
Table 4: Tableting speed study – Compression and tablet data – results shown for a 15 kN compression force (mean ± SD)
|
Tableting speed (tablets/min) |
20 |
40 |
60 |
|---|---|---|---|
|
Weight (mg) |
509.3 ± 0.80 |
508.1 ± 1.88 |
506.5 ± 0.63 |
|
Ejection force (N) |
166 |
207 |
230 |
|
Hardness (N) |
55.7 ± 0.95 |
51.0 ± 1.49 |
49.6 ± 1.17 |
|
Friability (%) |
0.99 |
1.05 |
1.26 |
|
Density (g/mL) |
1.246 |
1.223 |
1.219 |
|
Disintegration time (s) |
74 ± 22 |
79 ± 29 |
55 ± 16 |
The impact of compression settings on tablet properties were also studied using a Stylcam. This study was conducted to find the optimal tableting parameters for the formulation of small (8 mm) convex tablets of 150 mg at an industrial level (Figure 6). The Stylcam was set to simulate a rotary press (Fette P1200) at a production rate of 72,000 tablets/h. Among all the parameters that could be evaluated, the precompression and compression forces were the ones selected in addition to the impact of production rate.
Precompression influence
The influence of a precompression force on ODT performance can be seen in Figure 6, where the hardness and friability are shown as a function of precompression force (compression force 10 kN). Precompression forces of 0, 1.8, 3.2, 6.3 and 8.8 kN were applied followed by a compression force of 10 kN.
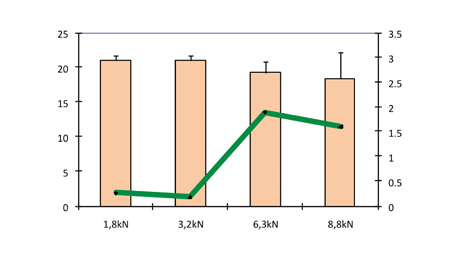
Figure 6: Hardness and friability as a function of precompression force (compression force 10 kN)
If no precompression force was applied, there was systematic occurrence of capping. This phenomenon was greatly reduced by the precompression step, which contributed to removing the air inside the powder. A precompression force from 1.8 to 3.2 kN was sufficient to prevent the occurrence of capping. Higher precompression forces above 6 kN generated a new capping.
The increase in compression force shown in Figure 7 did not result in any increase in hardness. Capping has been observed to occur above a compression force of 16 kN. Similar to the results obtained in the study conducted on larger sizes of tablet, in this study there was no effect by the compression force on disintegration time (Figure 8).
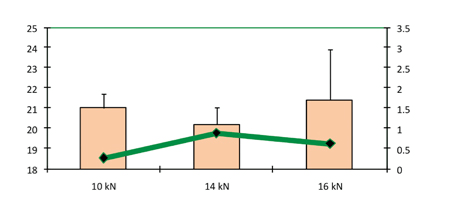
Figure 7: Hardness and friability as a function of compression force (precompression force 1.5 kN)
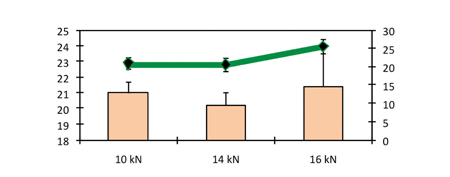
Figure 8: Hardness and in vitro disintegration time as a function of compression force (precompression force 1.5 kN)
We can confirm here that the mechanisms underlying the disintegration process with PEARLITOL® Flash are different from those reported with ODT disintegration.1,2 It seems that the mechanism of disintegration is not predominantly driven by the porosity of the tablet (Table 3). We hypothesize that the wettability properties of the mannitol and starch compound play a major role resulting in a quick absorption of water by the tablet.
Figure 8 shows the hardness and in vitro disintegration time as a function of compression force for a precompression force of 1.5 kN.
Production rates
Figure 9 shows the hardness and friability as a function of production rate using a precompression force of 1.8 kN and compression force of 10 kN. Production rates of 28,800, 86,400, 120,000 and 144,000 tablets/h were evaluated.
At speeds of up to 120,000 tablets/h, there was practically no influence by production rate on the hardness and friability of the tablets. At 144,000 tablets/h, capping was generated, reflected by the high variations in friability and disintegration times. The production rate had no influence on the disintegration time of tablets. This can be seen in Figure 10, which plots the hardness and in vitro disintegration time as a function of production rate using a precompression force of 1.8 kN and compression force of 10 kN.
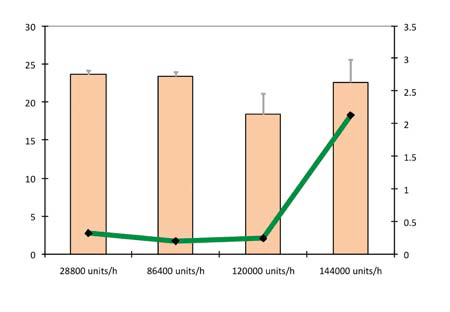
Figure 9: Hardness and friability as a function of production rate (precompression force 1.8 kN and compression force 10 kN)
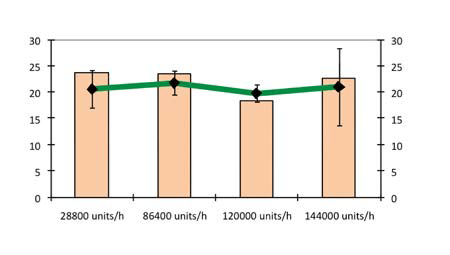
Figure 10: Hardness and in vitro disintegration time as a function of production rate (precompression force 1.8 kN and compression force 10 kN)
Conclusion
In conclusion, PEARLITOL® Flash has been developed as a self-disintegrating mannitol compound for the formulation of ODTs by direct compression. Irrespective of what size of tablet was chosen (13 mm or 8 mm), mechanically stable tablets were obtained for compression forces of around 14-15 kN. The Stylcam simulator has allowed us to find the optimal settings for small sized tablets with a convex shape. A precompression step has been identified as necessary to limit the occurrence of capping. Thanks to the specific composition of PEARLITOL® Flash, only a very low level of lubricant is required (approximately 0.4-0.5%).
This study also revealed that the disintegration time could not be correlated to the compression force, nor the hardness or the tableting speed. To conclude, PEARLITOL® Flash fulfills the requirement of excipients used to formulate ODTs in terms of flowability, hygroscopicity, compactibility, and disintegration.
References
- Zhao N., Augsburger L.L. AAPS PharmSciTech 6(1): Article 19 (2005).
- Carter J.C. Pharmaceutical Canada, 3, 2, 1-4 (2002).
- Harmon T. Tablets & Capsules, 1-5 (2006).
- Bhowmik D., Krishnakanth C.B., Chandira P.R.M. J. Chem. Pharm. Res., 1(1):163-77 (2009).
- Shaikh S., Khirsagar R.V., Quazi. Int. J. Pharmacy Pharm. Sci., 2(3) 9-15 (2010).
- Gupta A, Mishra AK, Gupta V., Bansal P., Singh R. Singh AK. Int. J. Pharm. Bio. Arch., 1(1):1-10 (2010).
- Wagh M.A., Dilip K.P., salunkhe K.S., Chavan N.V., Daga V.R. Int. J. Drug Del, 2:98-107 (2010).
- Balasubramaniam J and Bee T. Pharm. Tech. (Suppl. April 2009).
- Singh S.S., Mishra D.N., Jassal R., Soni P. Asia. J. Pharm. Clin. Res., 2(3):74-82 (2009).
- Shahi S.R., Agrawal G.R., Shinde N.V., Shaikh S.A., Shaikh S.S., Somani V.G., Shamkuvar P.B., Kale M.A. Rasayan J. Chem, 1(2):292-300 (2008).
- Chaudhary S.A., Chaudhary A.B., Mehta T.A. Int. J. Res. Pharm. Sci., 1(2):103-07 (2010).
- Yoshinari T., Forbes R.T., York P. Kawashima Y. Int. J. Pharm., 247: 69-77 (2002).
The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission