Robust Tableting Excipient: Starch-Lactose Compound
Situation
Both starch and monohydrate lactose are two traditional and well-established excipients used in numerous drug preparations. The combination of these excipients needs a wet granulation prior to tableting, due to segregation tendencies and poor powder flow. On the opposite the co-processed starch-lactose compound StarLac® (Meggle GmbH, Germany and Roquette Frères, France) is an excipient perfectly adapted to direct compression by combining superior tableting properties with high disintegration power. It is obtained by co-spray drying of 85% monohydrate lactose and 15% of white maize starch. StarLac® has been designed to reach in all conditions optimal tablet disintegration. In addition, several authors1, 2 have described important improvements of tableting parameters, such as reduced ejection forces, compared to physical blends of lactose and starch.
Challenge
The aim of this work was to check the robustness of StarLac® when used as a general DC excipient by testing it on a compression simulator STYLCAM 200 R and varying crucial tableting parameters.
Solution
Materials
Coprocessed starch-lactose compound StarLac® from Roquette Frères (Lestrem, France) has been used. Magnesium stearate came from Bärlocher GmbH.
STYLCAM 200 R simulator is a single punch equipment designed to simulate industrial rotary tablet presses. Contrary to usual single punch press, STYLCAM is doing a precompression, a symmetric compression and it has the same velocity as an industrial rotary press. The punches are equipped with strain gauges and displacement transducers giving access to compression parameters and to powder behavior under compaction.
Tableting studies
StarLac® was lubricated with 0.5% Magnesium Stearate (Turbula Mixer, W.A. Bachofen AG, Switzerland) for 5 min before compression. All tableting trials were carried out on a compression simulator STYLCAM 200 R, using Ø 10mm Euro BB punches of the type D10R9 and under controlled climatic conditions (temp. 22°C ± 2°C and humidity 44% ± 4%). The mean tablet weight was 450 mg.
The STYLCAM compression parameters were fixed in order to mimic the technical profile of a Fette P2090i press. The basic compression conditions were systematically varied:
- Compression force: 5 kN, 10 kN, 15 kN and 20 kN with a precompression of 0% or 20% (additionally precompression of 30% or 40% at 20 kN compression force).
- Tableting speed: 10, 25 or 40 tablets/ min, corresponding to 40500, 101250 and 162200 tablets/ hour on a Fette P2090i press.
Tablet properties
Tablet hardness was measured using a Dr. Schleuniger Pharmatron Tablet Tester 8 M (Sotax, Switzerland). Measurements were carried out on 10 tablets per compaction point and results were expressed as the mean value +/- standard deviation.
Tablet friability was measured using an Erweka Friabilator TAR following the European Pharmacopeia 8.0, 01/2010:20907.
Tablets disintegration was measured using a Pharmatron DisiTest 50 (Sotax, Switzerland) following the European Pharmacopeia 8.0, 01/2011:20901.
Results
StarLac® turned out to be a very robust binder–disintegrant under different concrete compaction situations. Modulating the compression speed (see figure 1) had no influence on the obtained tablet hardness.
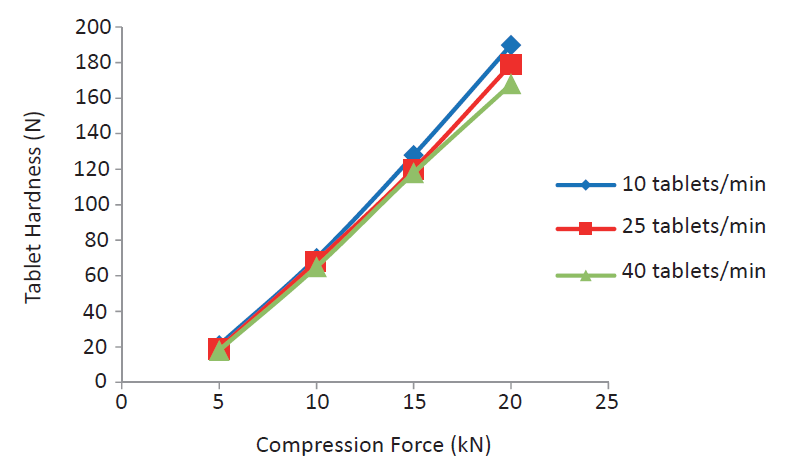
Figure 1. No impact of the tableting speed on the hardness of StarLac® tablets. The graph shows values for tableting with 20% precompression.
Identical tablet hardness was obtained when working with moderate or consequent precompression. At constant production speed, the precompression alteration had minor influence on the obtained tablet hardness (see figure 2).
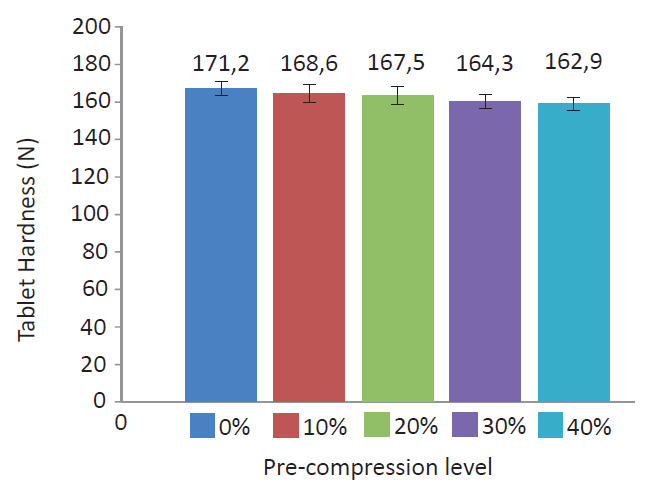
Figure 2. Low impact of the precompression level on the hardness of StarLac® tablets. The compression velocity was 40 tablets per min and compression force 20 kN.
The standard deviation of hardness remained very low at any compression force and tableting speed, with and without precompression. That confirms that there was no capping or beginning of capping although high concavity (D10R9) punches were used.
Modulating the precompression rates had only minor influence on the friability of the obtained tablets which is very low from 10 kN up to 20 kN (see figure 3).
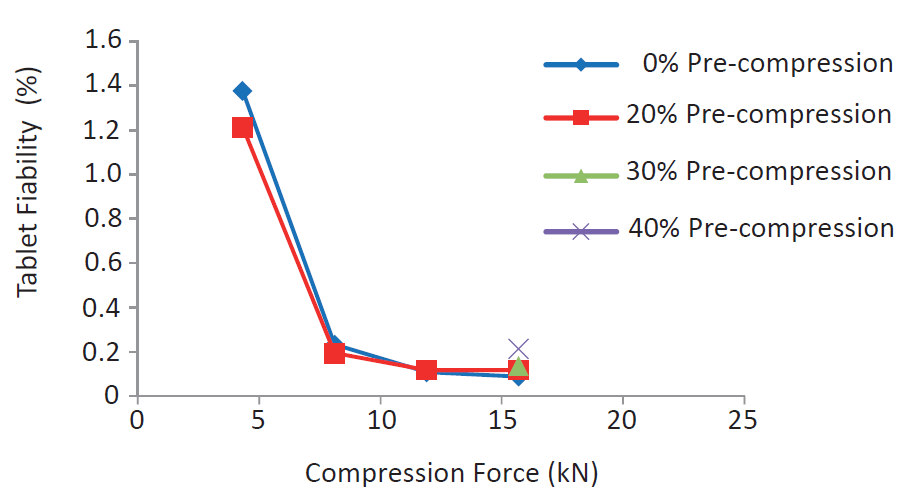
Figure 3. Friability of StarLac® tablets at different compression forces, using different precompression ratios. The tableting speed was 40 tablets per minute.
Nevertheless, combining high precompression rates with important compression forces could weaken the tablet, due to the presence of an elastic starch phase. At important compression forces, a limited increase of the tablet friability (from 0.03% to 0.16%) was detectable when increasing the precompression from 10% to 40%.
The disintegration time of tablets was always very low confirming the high propensity for disintegration of StarLaC®. It increased slowly with the compression force increase. Surprisingly, this disintegration time was higher when precompression was used. It may be due to a higher homogeneity of the tablets (see figure 4).

Figure 4. Disintegration time of StarLac® tablets at different compression forces. The tableting speed was 40 tablets per minute.
Conclusion
StarLac® is a robust DC excipient. The tablet properties (hardness, friability, disintegration) were hardly influenced by production parameters. This low impact of tableting parameters ensures an easy transfer from pilot plan to industrial production avoiding associated scale problems. StarLac® could be considered as perfect filler–binder for direct compression applications, combining fast tablet disintegration with easy tableting.
References
- Wagner, K.G. et al.: A Corn starch /α-Lactose Monohydrate Compound as a new Directly Compressible Excipient, Pharm. Ind. 2002; 64(9): 992-99.
- Häusler, O.: STARLAC® – A Star is Born; Pharmaceutical formulation and quality (6,7) 2002; 54-57 (see also http://www.roquette-pharma.com/2002/starlacsupsup-a-star-is-born).
- Hauschild, K et al.: Evaluation of a new coprocessed compound based on lactose and maize starch for tablet formulation, DOI: 10.1208/ps060.
The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission.