PEARLITOL® 200 GT Mannitol: Harnessing the Potential of Higher Active Ingredient Content for Smaller, More Convenient Tablets
Published February 20, 2026
Authors
Philippe Lefèvre
Head of Global Pharma Applications Labs, Roquette
Nicolas Descamps
Manager of the Functional Properties Analytical Laboratory, Roquette R&D
Sébastien Croquet
Application Scientist, Roquette
Introduction
Mannitol is the first intention excipient for oral solid forms, designed for APIs having stability problems. Mannitol is not hygroscopic and presents a high chemical stability; therefore, it is considered compatible with almost all drugs.
To combine its high stability properties with a cost-effective production process, textured mannitol powders have been developed for the manufacturing of tablets by direct compression.1
During tablet production, capping may occur depending on the API properties and ratio, the tablet shape (as convex tablets), the compression pressure, and the tablet production speed. Several solutions are proposed to decrease or suppress capping, as precompression step, lower production speed, lower compression force. But often, it appears necessary to decrease the API content to produce conform tablets at an acceptable production output.
PEARLITOL® 200 GT is a new pure beta mannitol grade for direct compression which powder properties have been optimized to limit the occurrence of capping. It was demonstrated that the high powder density of this new and innovative directly compressible mannitol explains its unique behavior of suppressing capping or moving it to higher compression force and/or higher tableting speed.2
OBJECTIVES
This study aimed to demonstrate that PEARLITOL® 200 GT gives effectively access to higher API content, and consequently smaller tablet size, compared to two well-known directly compressible mannitol products: Mannitol SD-A, a spray-dried mannitol and Mannitol G-B, a granulated mannitol.
Sitagliptin was used as a drug model as its powder properties favors the occurrence of capping. The objective was to formulate tablets of 400 mg weight and 100 N hardness.
A compression simulator STYLCAM 200R from MEDELPHARM (France) was used, and a production at 140,000 tablets/hour on a KORSCH XL400 was simulated. Increased ratio of sitagliptin were tested until capping was observed.
Materials and Methods
Materials
PEARLITOL® 200 GT mannitol from Roquette Frères (Lestrem, France)
Mannitol SD-A: spray-dried mannitol
Mannitol G-B: low density granulated mannitol
Sitagliptin phosphate monohydrate powder (Starpharm, China)
Vegetal magnesium stearate (Roquette Magnesium Stearate)
Methods
Blending
Mannitol and sitagliptin were mixed for 5 min in a Turbula mixer (WAB-Group, Muttenz, Switzerland). Additional mixing for 5 min was done after adding the magnesium stearate.
Powder characteristics using the TADES (tabletability development system) spider plot3:
Eight powder properties are measured and regrouped into four categories, each category corresponding to an important step in the overall powder tabletability. For each property, the measure is expressed on a 0 to 10 scale, and all results are presented on a spider chart. It is anticipated that to be workable on a high-speed rotary machine, the powder must have a value higher than 5 for all the required properties (red dotted line on the graph)
Tableting
All tableting trials were done on rotary tablet press simulator STYLCAM 200R (MEDELPHARM), mimicking KORSCH XL400 Euro B.
Punches: Euro B Elizabeth D10R10
Die: Euro B Elizabeth D10
Tablet weight: 400 mg
Compression force: 10, 15, 20, 25 kN
Precompression force: 0 kN
Tableting speed: 40 tablets per minute (equivalent to a rotary press speed of about 140,000 tablets/hour).
Tablet characterization
All tablets were visually inspected; capped tablets are recorded without any further characterization. Per test, 30 tablets were analyzed on their dimensions, weight, and hardness, using a Pharmatron ST 50 (Solothurn, Switzerland) equipment.
Results And Discussions
The TADES powder properties quotation system was used to predict the behavior of the blends in the tablet press and assure that the feeding was good enough to produce tablets with constant properties.
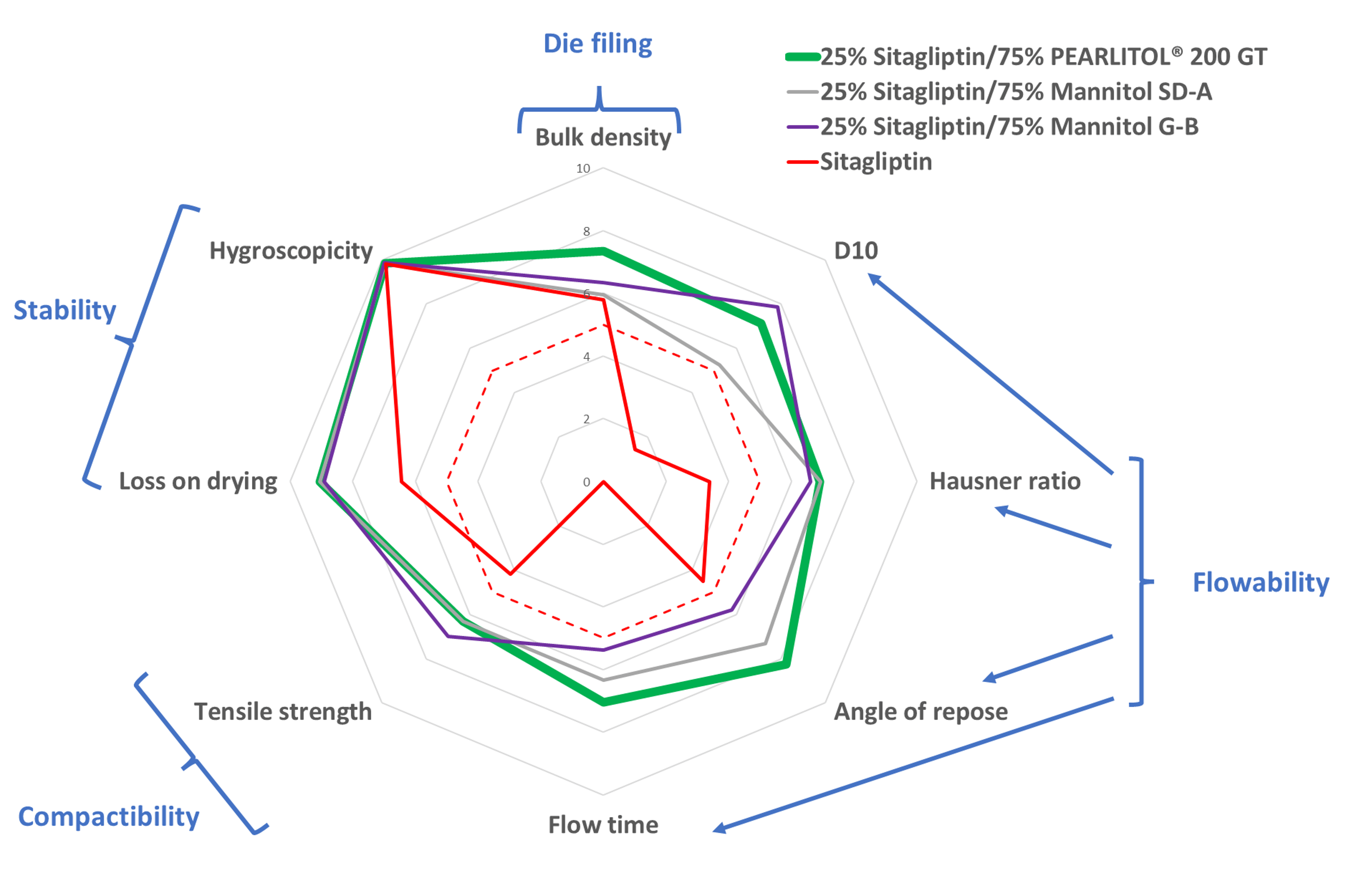
Sitagliptin itself has poor powder properties (see fig. 1) and is not usable on a rotary press. Indeed, it was not possible to produce any tablet on the compression simulator. All blends with 25% sitagliptin and mannitol present powder properties ensuring stable tablet production (all properties' quotations being higher than the limit of 5, represented by the red dotted line). PEARLITOL® 200 GT presents the best behavior, with all properties’ quotations being higher than 6.
Figure 1. Prediction of the blends’ behavior on tablet press feeding using the TADES quotation – sitagliptin alone or blends with 25% sitagliptin and 75% of the named mannitol.
Tableting studies demonstrated real difference between the three mannitol powders tested.
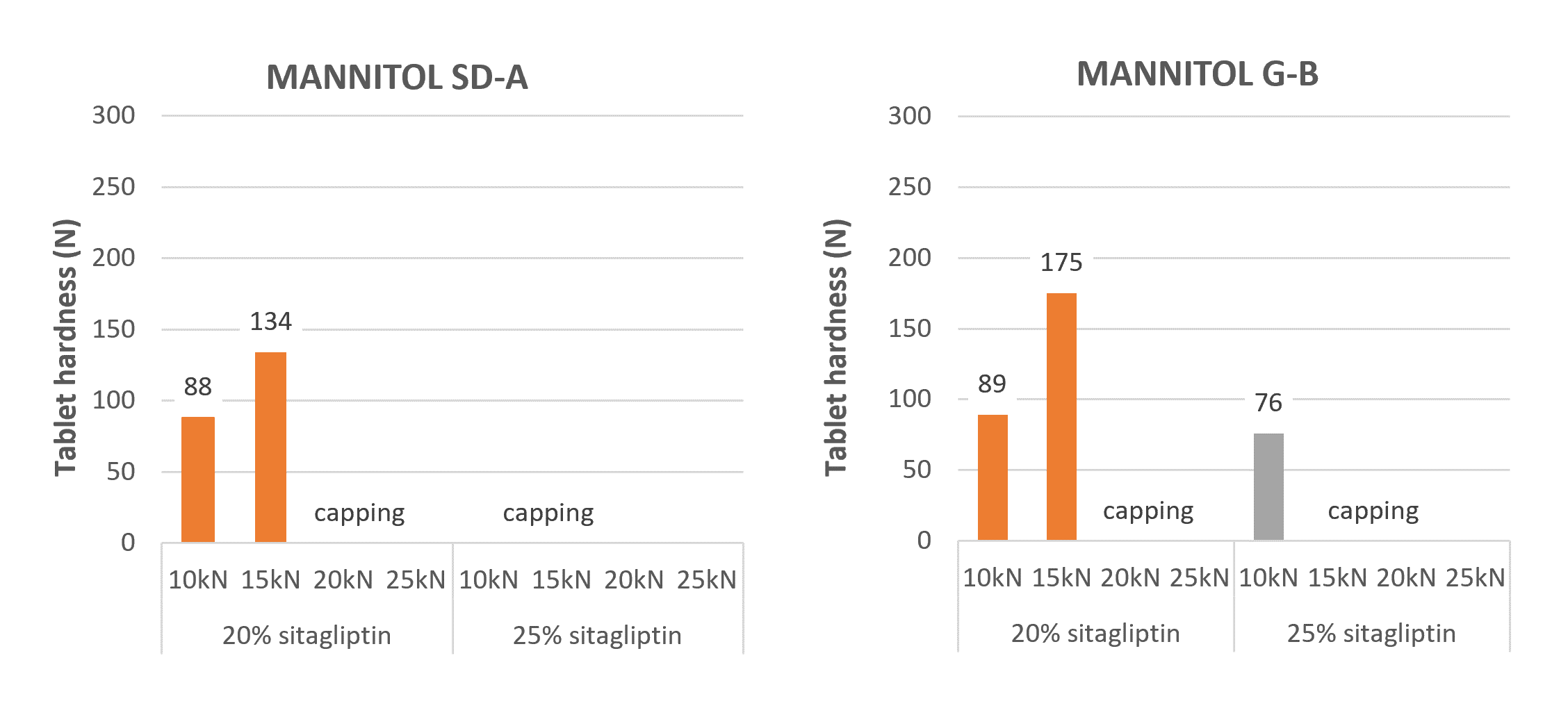
With Mannitol SD-A, spray-dried mannitol powder, it was not possible to obtain tablets with 25% sitagliptin, whatever the compression forces, due to the occurrence of capping (fig. 2). With 20% sitagliptin, 134 N tablet hardness was obtained at 15 kN compression force while capping was observed at higher compression force.
With Mannitol G-B, low density granulated mannitol, the maximum tablet hardness reachable with 25% sitagliptin was 76 N, so under our tablet hardness specification of 100 N. Then capping was observed. As with Mannitol SD-A, 20% sitagliptin tablets were obtained (175 N) at 15 kN compression force, and capping was observed at higher compression force.
Figure 2. The tableting results with the Mannitol SD-A and Mannitol G-B.
The behavior of the blends of sitagliptin and PEARLITOL® 200 GT is completely different than what was observed previously.
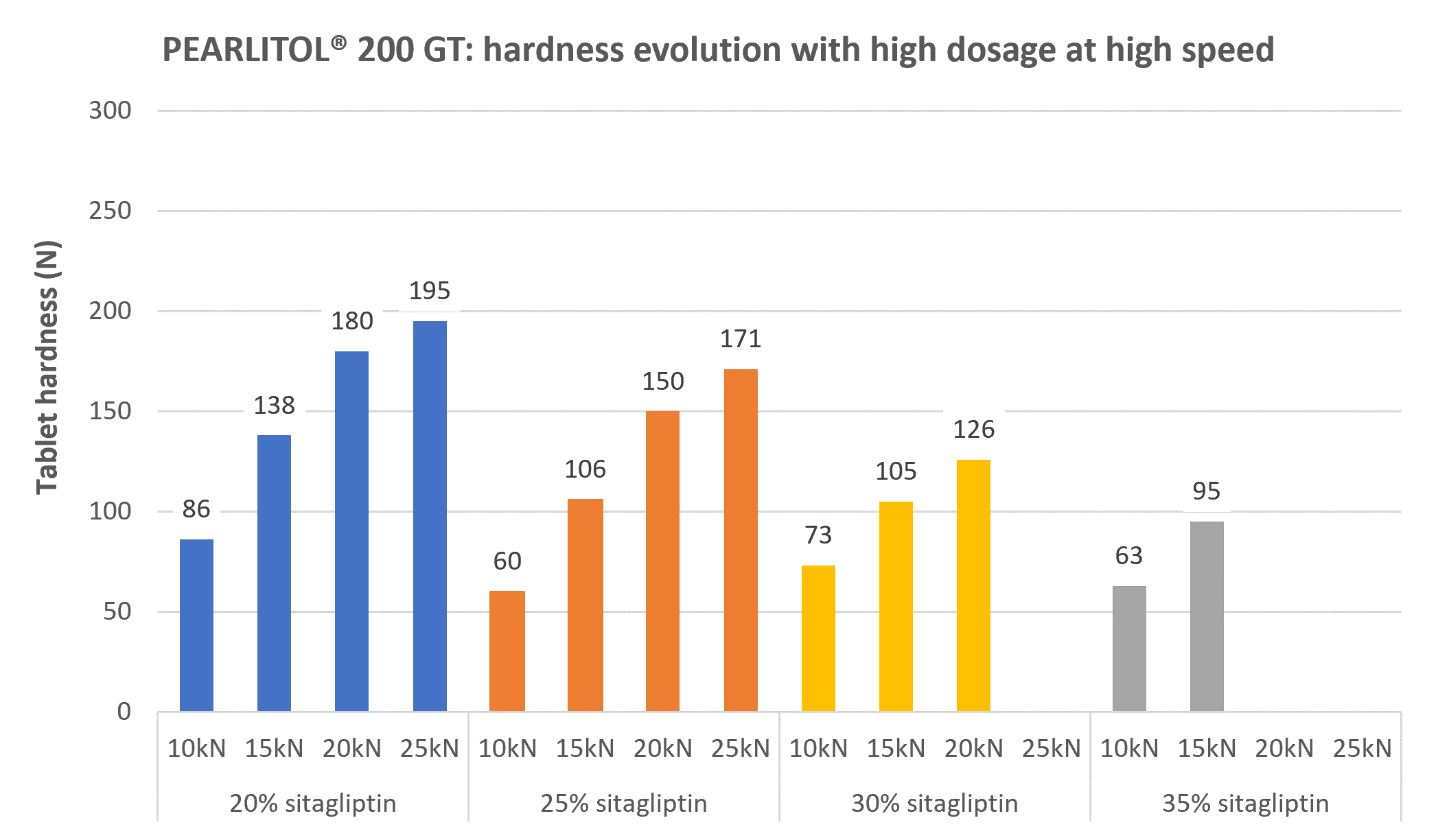
With 20% and 25% sitagliptin, the maximum compression force authorized by the size and shape of the punches (25 kN for D10R10 punches) was reached without capping.
With 30% sitagliptin, 126 N tablet hardness was obtained at 15 kN compression force while capping was observed at higher compression force.
With 35% sitagliptin, capping appeared also upper 15 kN. But due to the no compactibility of sitagliptin and its high incorporation level, tablets done at 15 kN were not hard enough (95 N) regarding our specification of 100N.
Figure 3. The tableting results with PEARLITOL® 200 GT.
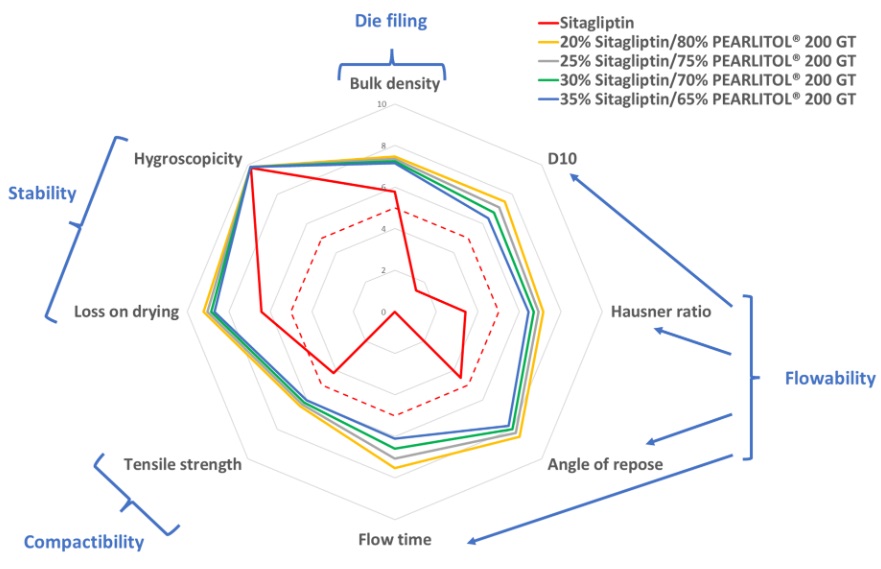
The TADES powder properties quotation system was performed to ensure that the properties of the blends with increasing sitagliptin ratio were still good enough to produce tablets with constant properties (fig. 4). Even with 35% sitagliptin, all TADES selected properties are superior or equal to 5, the limit for an acceptable powder behavior. It confirms that the low tablet hardness observed with 35% sitagliptin is really due to the non compactibility of this API and is not linked to insufficient powder properties of the blend.
Figure 4. Prediction of the blends’ behavior on tablet press feeding using the TADES quotation – sitagliptin alone or blends with 20%, 25%, 30% and 35% sitagliptin and PEARLITOL® 200 GT.
Conclusion
This real case with sitagliptin tablet formulation and simulating a KORSCH XL 400 tablet press running at 140 000 tablets/hour demonstrates the benefits of using PEARLITOL® 200 GT. While the maximum sitagliptin incorporation rate was 20% with today’s most used directly compressible mannitol, Mannitol SD-A and Mannitol G-B, an incorporation rate of up to 30% was accessible with PEARLITOL® 200 GT. This API content increase presents many benefits for the formulators and the patients (table 1). For the same tablet weight, it allows increasing the API content by 50% and limits the number of tablets per day. Or, for the same API content, it allows a lower weight of 33% for an easier uptake.
Table 1. Impact of API ratio increase on API content or on tablet weight.
| Mannitol SD-A | Mannitol G-B | PEARLITOL® 200 GT | PEARLITOL® 200 GT | PEARLITOL® 200 GT | |
| Sitagliptin ratio | 20% (maximum) | 20% (maximum) | 20% | 25% | 30% (maximum) |
| Same tablet weight 400 mg | 80 mg | 80 mg | 80 mg | 100 mg | 120 mg |
| API dosage increase | + 25% | + 50% | |||
| Same sitagliptin content 80 mg | 400 mg | 400 mg | 400 mg | 320 mg | 267 mg |
| Tablet weight decrease | - 20% | - 33% |
References
1. Boldhuis G.K., Rexwinkel E.G., Zuurman K., Polyols as filler-binders for disintegrating tablets prepared by direct compaction, Drug Dev. Ind. Pharm., 35,671-677 (2009)
https://doi.org/10.1080/03639040802587799
2. Lefèvre, P., Haeusler, O., Boit, B., Croquet, S., Comparison of capping tendency of directly compressible mannitol powders, Poster, 4th European Conference on Pharmaceutics, 20-21 March 2023, Marseille, France.
3. Descamps, N., Lefèvre, P., Hu, E., Popescu, C., Tablet powder mix computational physics requirements to be processed by direct compression, Poster, 13th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, 28-31 March 2022, Rotterdam, Netherlands.
https://doi.org/10.13140/RG.2.2.10196.78725
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.