PEARLITOL® 200 GT Mannitol: A Tool to Formulate Mini Tablets as a Compliant and Flexible Pediatrics Delivery System
Presented at the AAPS 2023 PharmSci 360, October 22-25, 2023, Orlando, Florida under the title “Mini Tablets are a Compliant and Flexible Pediatrics Delivery System”
PURPOSE
Mini tablets are a multiple unit dosage form with a size of less than 3.0 mm in diameter with great flexibility to deliver appropriate doses to the pediatric age group. They can be given in different numbers, according to dosage requirements, with no special prerequisite of formulating age group customized dosage tablets. Mini tablets are a friendly drug delivery system for patients having impaired swallowing issues with a polypharmacy therapy. The objective of the study was to develop melatonin (as a model drug) mini tablets, by direct compression, for pediatrics to replace conventional size tablets at the same strength of dose.
MATERIALS AND METHODS
Materials
Melatonin (BOC Science) as model drug.
PEARLITOL® 200 GT mannitol (Roquette) as direct compression filler-binder.
GLYCOLYS® sodium starch glycolate (Roquette) as disintegrant.
Vegetal magnesium stearate (Roquette Magnesium Stearate) as lubricant.
Methods
Blending
Mini tablets of 3 mg melatonin were formulated according to table 1.
Table 1. Formulations of 3 mg melatonin mini tablets
| Ingredients | Formulation composition with disintegrant (%) | Formulation composition without disintegrant (%) |
| Melatonin | 20.00 | 20.00 |
| PEARLITOL® 200 GT | 75.39 | 78.53 |
| GLYCOLYS® | 3.14 | 0 |
| Magnesium stearate | 1.47 | 1.47 |
| Total | 100.00 | 100.00 |
Melatonin, PEARLITOL® 200 GT and possibly GLYCOLYS® were first blended in a TURBULA® mixer (WAB T2F) at 49 rpm for 5 min. The lubricant, magnesium stearate, was added to the blend and mixed at 49 rpm for 2 min. Approximately, 10 mg of sample blend was taken from three different spots for content uniformity evaluation.
Tableting
The powder mix was compressed using 2.5 mm standard concave punch (Natoli Engineering) single tip using STYL’One Evo compaction simulator (MEDELPHARM) simulating Korsch XL 400 tablet press. Formulation mix was compressed at four different compression forces (0.65 kN, 1.30 kN, 1.66 kN, and 2.10 kN) at 20 RPM (equivalent to a rotary press speed of about 42,000 tablets/hour) with a target mini tablet weight of 15 mg.
Tablets characterization
Breaking force was checked at each compression force using hardness tester. Disintegration of mini tablets was tested at 37°C in 800 mL of distilled water using Sotax DT50 disintegration tester. High performance liquid chromatography (HPLC-UV) using a detection wavelength of 277 nm was used to quantify melatonin content uniformity in powder blends and tablets, and to evaluate its dissolution profile. Conventional marketed regular size melatonin tablet at same drug content (3 mg/tablet) were used as reference for dissolution comparison (see figure 1).

Figure 1. Comparison of mini tablet (15 mg weight) and regular size tablet (300 mg weight) at the same dose strength of 3 mg of melatonin.
To reach within linearity range of HPLC method, three tablets are needed in each vessel. Dissolution of three tablets (9 mg melatonin) was done in each vessel (n=3) of 500 mL distilled water at 37 °C with a paddle speed of 100 rpm. Samples were taken at 5 min, 10 min, 15 min, and 30 min from each vessel and filtered using 0.20 µm nylon filter for melatonin quantification by HPLC.
RESULTS
The flowability of the blend was excellent, with a Hausner ratio of 1.11. The advantage of having excellent flow is indicated by the low weight variation of about 2% (10 mini tablets’ average weight was 15.4 ± 0.32 mg). Despite the small diameter die of 2.5 mm, mini tablets display weight consistency. The formulation blend displayed 99.5 % content uniformity, evaluated by HPLC, with a standard deviation of 0.45 %. In comparison, the weight variation of commercial melatonin tablets was higher (weight of three tablets from 902.91 mg to 934.28 mg).
Mini tablets formulated without disintegrant presented disintegration times of around 2-3 min at all compression forces. In addition, approximately 100 % melatonin was dissolved in 30 min (see figure 2) with more than 80% within 10 min.
Mini tablets formulated with disintegrant showed similar dissolution profile as the regular marketed control (see figure 2) with more than 80% drug released within 5 minutes.
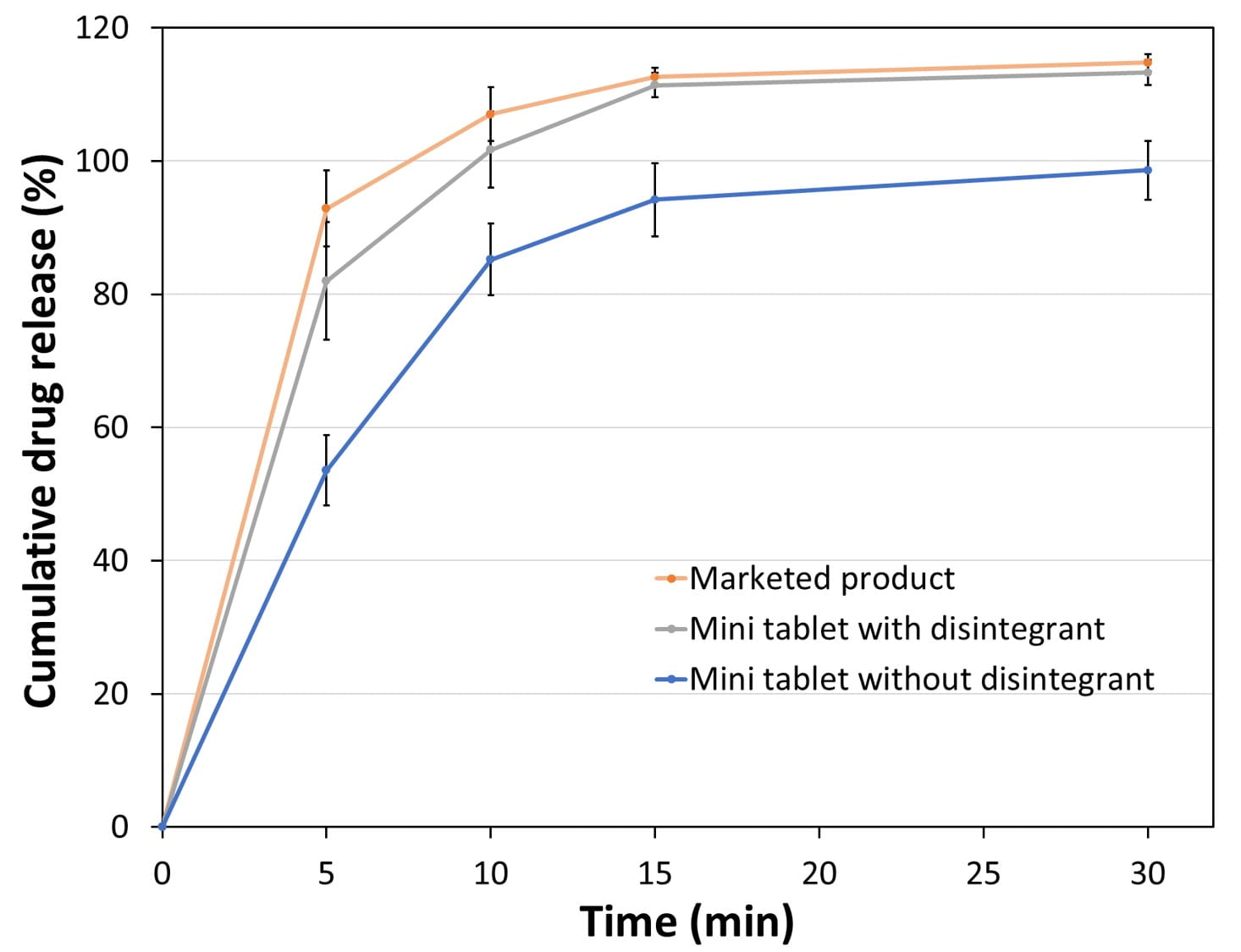
Figure 2. Dissolution profile of mini tablets (tablet weight: 15 mg) and marketed product (tablet weight: 300 mg), all having 3 mg melatonin potency.
CONCLUSION
Mini tablets of melatonin were successfully produced by direct compression with similar drug release kinetics compared to conventional marketed tablets.
PEARLITOL® 200 GT showed here excellent properties as filler for direct compression. Its good flowability enabled to produce mini tablets with a very low tablet weight variation, which is of high importance in such dosage form. In addition, its improved compressibility prevented capping ensuring it is the perfect choice for tablets of any size.
In addition, mannitol, being a water-soluble excipient yet not hygroscopic, contributes to increase tablet shelf life.
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.