Binder for continuous wet granulation: case of soluble system
Introduction
Twin-screw granulation is a modern and efficient tool for the continuous production of solid-dosage form. It permits high flexibility and adaptation to different powder systems. Common wet granulation binders present specific physical properties, offering the possibility to select the ones answering precisely to formulations’ needs. A rational binder selection improves the granulation process and the quality of the final product.
When a binder is added in a dry state, it needs to interact with the granulation liquid (e.g., water) to activate its binding properties. In a soluble or partially soluble formulation (containing soluble APIs and/or soluble excipients such as lactose or mannitol), the granulation liquid interacts also with the soluble parts of the formulation. Consequently, not all granulation liquid is available for the binder activation. However, soluble and dissolved parts of the formulation could contribute to the granule’s formation as well. Adjusting the binder properties (e.g., its dissolution speed and its viscosity) helps optimize these simultaneous physical effects (wetting, dissolution and crystallization) for the best possible granulation result.
As mannitol exhibits a high aqueous solubility and dissolution rate, it was chosen as a model for a completely soluble system. When drying, mannitol crystallizes rapidly and completely, without forming any amorphous or metastable phases. This contributes therefore to a stable process, adapted to a high throughput, as the total granulation and drying time last several minutes only.
The need for water for the full binder activation, expressed as liquid / solid ratio (L/S-ratio) is a good performance indicator for the granulation process. It witnesses the efficiency of each binder under concrete use conditions. A friability threshold of 30% was set as a limit above which granules were susceptible to attrition and breakage during downstream processes (Keleb 2002, Portier 2020, Vanhoorne 2016).
Materials and methods
Mannitol (PEARLITOL® 50C, Roquette Frères) was selected as model for a soluble formulation. The wet granulation (WG) binders, used in a concentration of 5%, are introduced via a prior dry-mixing step:
- Hydroxypropyl pea starch (LYCOAT® RS 720), Roquette Frères, France - (HP pea starch)
- Hydroxypropyl methylcellulose (METHOCELTM E15), Dow Chemical Company, Rheinmünster, Germany) - (HPMC E15)
All wet granulation binders were characterized using various physical methods. Powders were analyzed on particle size distribution, specific surface area, water-binding capacity (g of water/g of powder), and binder plasticity during tableting. Aqueous binder solutions (8% in water) were evaluated on the following criteria: wettability of solid surface (using drop shape analyzer applying the sessile drop method on mannitol tablets, and on Teflon and PTFE surfaces), surface tension and viscosity.
The granulation experiments were performed using the twin screw granulator of a continuous from-powder-to-tablet line (ConsiGmaTM-25 system, GEA Pharma Systems, Wommelgem, Belgium). This continuous granulator consists of two co-rotating screws with a length-to-diameter (L/D) ratio of 20/1. Screw speed and throughput were set on 300 rpm and 20 kg/h respectively.
After tray drying, the granules friability was measured using a friabilator, at a speed of 25 rpm for 10 min, by subjecting 10 g of granules together with 200 glass beads (mean diameter 4 mm) to falling shocks. The percentage of created fines (under 250 µm) was used to calculate the friability.
For the tableting trials, granules produced at an L/S-ratio of 0.08 were selected. The powder fraction 150-850 µm was used, after calibration on 1500 µm and sieving. The tableting (10 mm flat punches, tablets weight 400 mg, lubrication 0.7% Mg Stearate) was done with a tablet press simulator (STYLCAM200 R, Medelpharm, Beynost, France).
All data were treated via a principal component analysis SIMCA® 16 software (Sartorius Stedium Biotech, Umeå, Sweden). A score scatter plot of principal component analysis (not shown) permits to cluster binder in function of their effectiveness.
Results
Figure 1 represents the friability of granules obtained using different binders (HP pea starch and HPMC) as a function of the L/S ratio. Clear differences between the binder activities were observed, on the L/S- ratios required to obtain acceptable granules. For each binder, higher L/S-ratios resulted in less friable granules. This was attributed to more material dissolving in the granulation liquid, forming more solid bonds upon recrystallization during drying.
The highest binder effectiveness was found for HP pea starch, as this binder required the lowest amount of liquid to meet the friability limit (L/S-ratio of 0.0675). HPMC E15 needed much higher L/S ratios for effective granulation (required L/S-ratio of 0.1050). However, globally, including other polymers (see publication) only a limited difference in binder effectiveness of various tested polymers were found. This could be explained with the high aqueous solubility of mannitol, allowing this excipient to participate in the granulation process, contributing to bond formation within the granule.
Using blank water, granulated mannitol could be successfully obtained, but only under specific conditions. Too low L/S ratios (<0.08) caused machine blockage, because the barrel fill of the limitedly wetted powder bed was too high. At higher L/S-ratios (0.08), a strong bond formation was obtained with pure mannitol, as the granule friability was already below the 30% friability limit. The use of a higher L/S-ratio did not result in further lowering of granule friability. On the other hand, using additional wet granulation binder contributed to further reduction of the granule’s friability.
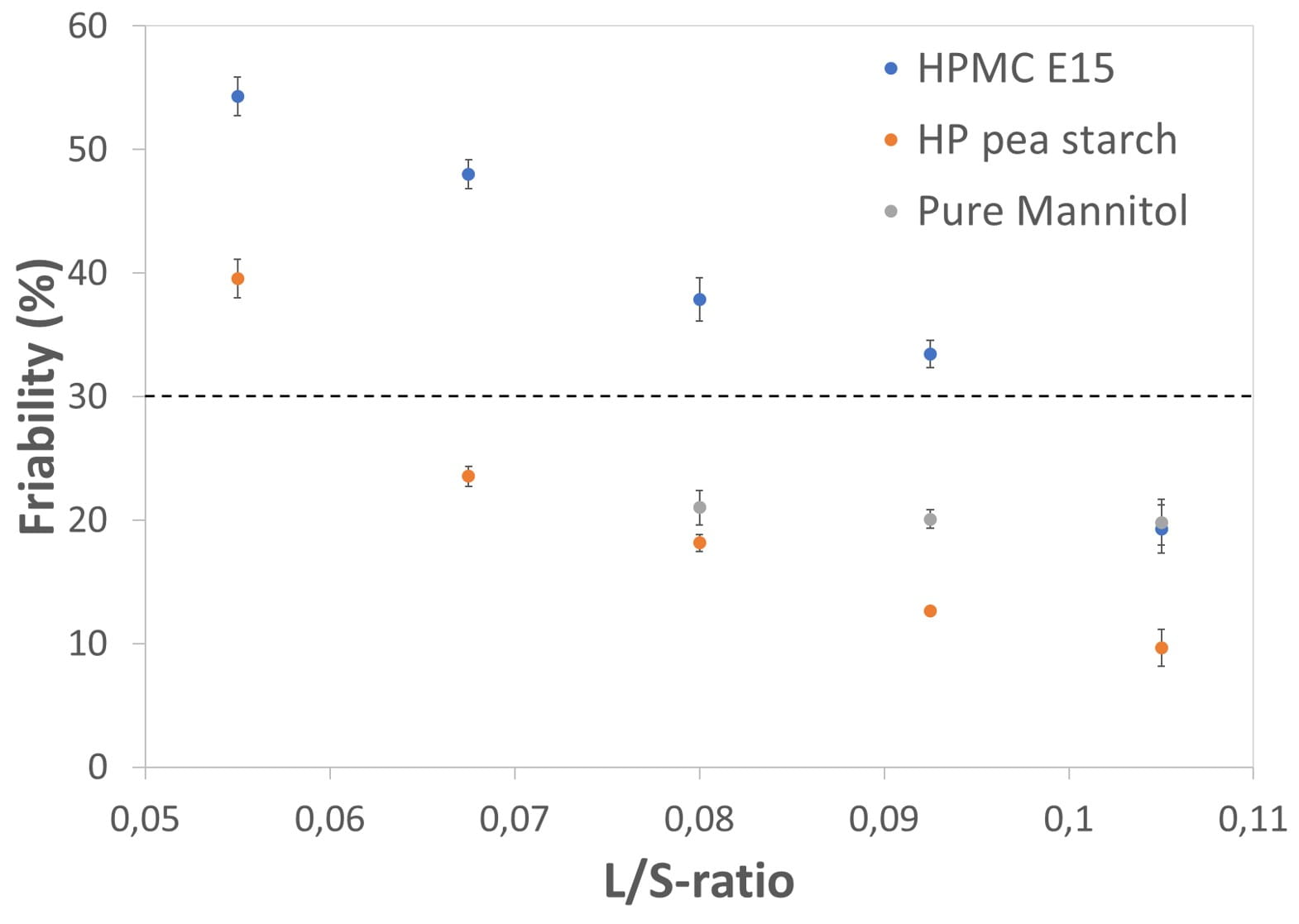
Figure 1. Granule friability in function of binder type and L/S-ratio. The dotted line represents a granule friability of 30%, set as maximum threshold to identify granules having sufficient strength.
Linking the binder properties with the result of the granulation trials via principal component analysis pointed out opposite product properties for HP pea starch and HPMC E15.
HP pea starch dissolves rapidly due to its good wetting capacity, resulting in rapid activation of its binding properties. When activated, the dissolved binder wets the (remaining) dry particles of mannitol efficiently. Low contact angles (CAs) on mannitol surfaces were measured with HP pea starch in solution.
In contrast, a minor binder activation was found for HPMC E15. This is due to its slow dissolution kinetics combined with higher viscosity and slower wetting. In addition, HPMC E15 wets mannitol surfaces to a much lower extent. The contact angles of 8% HPMC E15 solutions on mannitol tablets are high, with about 70° immediately and > 50° after 30 seconds. In comparison, contact angles on mannitol tablet for HP pea starch solution are 44° immediately and 33° after 30 seconds. This limits the formation of solid bridges between all mannitol particles, considering that the effective granulation time is about 5-20 seconds.
Tableting studies presented in figure 2 reflect the differences in binder efficiency.
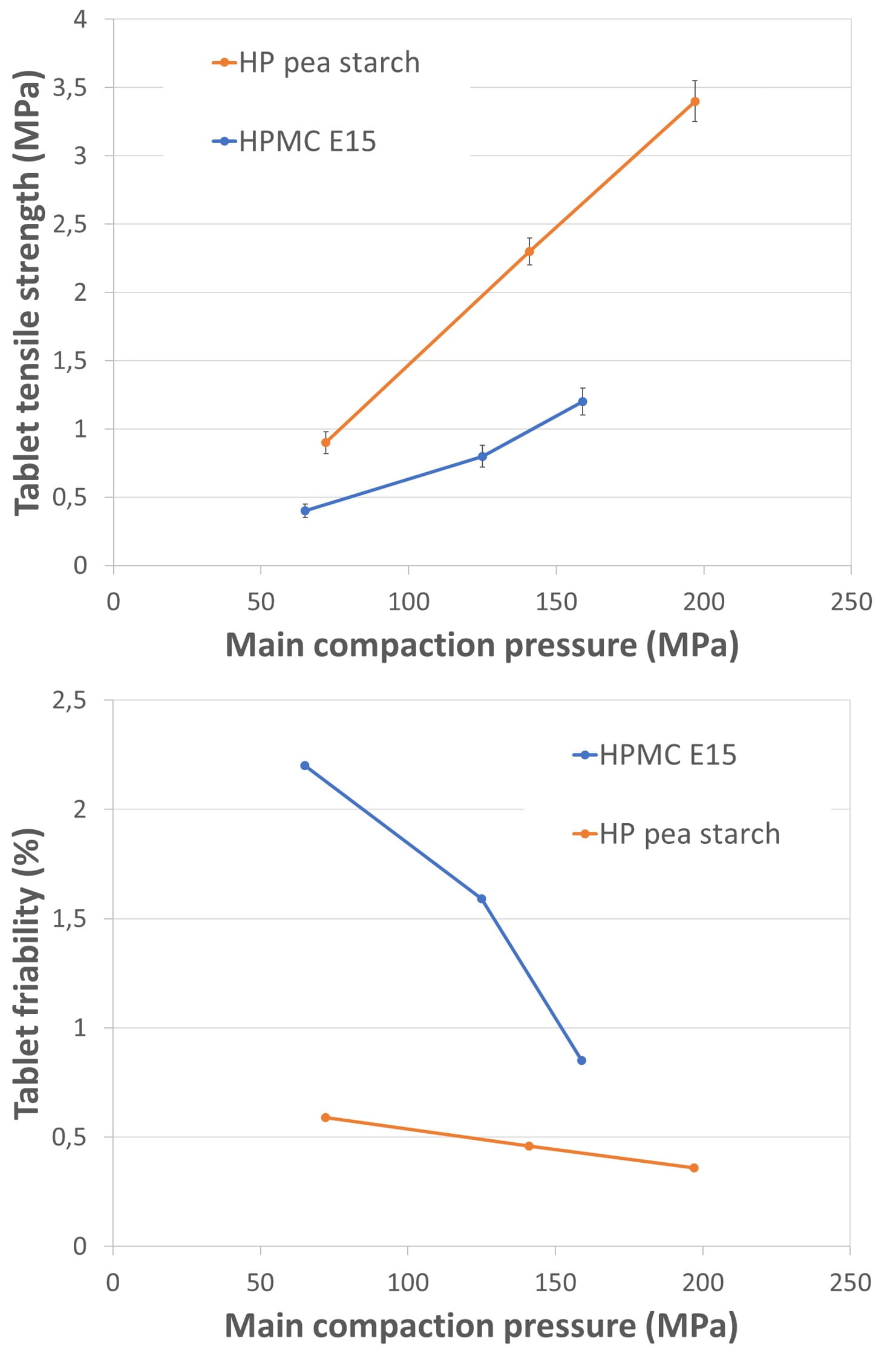
Figure 2. Results of tableting trials with mannitol granules, obtained with a L/S-ratio of 0.08. The graphs show the found tablet tensile strength and the tablet friability as a function of the main compaction pressure.
The granules obtained with HPMC E15 had lower powder flow, even when their particle size was comparable with that obtained from HP pea starch as binder. HPMC E15 formulation showed therefore a large variability in tableting because of inconsistent die filling. The tablets had too low tensile strengths (<1 MPa) for being acceptable. This poor tableting behavior of HPMC E15 granules might be explained with the binder’s lower plasticity factor (see table 1). Less plastic deformation is inherently linked to a decrease of particle bonding areas, negatively affecting compact formation with sufficient inter-particular bonds [Grymonpré, W.; et al].
Table 1.Plasticity factor of the pure binders
|
Binder Plasticity Factor (%) |
|
|
HPMC E15 |
88.01 (±0.08) |
|
HP pea starch |
96.38 (±0.05) |
Conclusion
In a continuous wet granulation process, using a wet granulation binder is of benefit, also in completely soluble systems. It renders the production process easier and stabler. Binder contributes to the formation of high-performing granules and hence harder tablets. The needed water quantity as binding liquid is significantly lower than in insoluble systems (see case study of insoluble system – insert link to case study to be published), because soluble powder contributes with its own binding capacity to the bonds formation.
The good performance of a wet granulation binder in mannitol-based formulations is linked with a fast activation of the binder properties. Differences in the mannitol wetting capacity of binders’ solutions contribute largely to the observed differences in granules and tablet properties.
For more details, see the following article:
Vandevivere, L., Vangampelaere, M, Portier, C., de Backere, C, Häusler, O.,
De Beer, Th., Vervaet, Ch., Vanhoorne, V.
Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process.
Pharmaceutics 2021, 13, 210.
https://doi.org/10.3390/pharmaceutics13020210
Further reading:
Keleb, E.I.; Vermeire, A.; Vervaet, C.; Remon, J.P. Continuous twin screw extrusion for the wet
granulation of lactose. Int. J. Pharm. 2002, 239, 69–80,
https://doi.org/10.1016/S0378-5173(02)00052-2.
Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin
screw granulation of controlled release formulations with various HPMC grades. Int. J. Pharm.
2016, 511, 1048–1057,
https://doi.org/10.1016/j.ijpharm.2016.08.020.
Vanhoorne, V.; Vervaet, C. Recent progress in continuous manufacturing of oral solid dosage forms.
Int. J. Pharm. 2020, 579, 119194,
https://doi.org/10.1016/j.ijpharm.2020.119194.
Portier, C.; Pandelaere, K.; Delaet, U.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: A complex interplay between formulation properties, process settings and screw design.
Int. J. Pharm. 2020, 576,119004,
https://doi.org/10.1016/j.ijpharm.2019.119004.
Grymonpré, W.; Verstraete, G.; Van Bockstal, P.J.; Van Renterghem, J.; Rombouts, P.; De Beer, T.; Remon, J.P.; Vervaet, C. Inline monitoring of compaction properties on a rotary tablet press during tablet manufacturing of hot-melt extruded amorphous solid dispersions. Int. J. Pharm. 2017, 517, 348–358,
https://doi.org/10.1016/j.ijpharm.2016.12.033.