Benefits of an Optimal Tabletability DC Mannitol on Compression Setting Parameters
Presented at the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, 18 - 21 March 2024, Vienna, Austria.
INTRODUCTION
An increasing number of APIs present stability challenges and corresponding tablets require to be formulated with protective excipients. Indeed, mannitol is the best alternative to promote tablet stability as it is non hygroscopic, possesses a high purity and chemical stability, and is considered compatible with almost all drugs.
To simplify and decrease the cost of tablet production, textured mannitol powders have been developed for the manufacturing of mannitol-based tablets by direct compression.1 During tablet production, many defects may occur, of which capping, function of the API properties and ratio, the tablet shape (e.g., convex tablets), the compression pressure, and the tablet production speed.2 Several solutions are proposed to decrease or suppress capping. The preferred one is to apply a precompression step. Other possible solutions are to decrease the production speed or the API load, having negative impacts on respectively cost of good or tablet size.
Finally, tablet capping is often linked with the need to reach tablet hardness target, while using high compression forces. The use of high compression forces or multiple compression steps (precompression) increases mechanical stress and consequently wear of punches.
OBJECTIVES
This study aimed to demonstrate that tablets can be produced without high compression forces or precompression when using a new direct compression (DC) mannitol grade, PEARLITOL® 200 GT, which powder properties have been optimized to improve tabletability and limit the occurrence of capping.3 First, the most frequently used DC mannitol, PEARLITOL® 200 SD, was formulated with a highly challenging API, sertraline, to demonstrate the need of high compression forces/precompression and then PEARLITOL® 200 GT was tableted in the same conditions.
MATERIALS AND METHODS
Materials
Granulated mannitol: PEARLITOL® 200 GT (GTman), spray-dried mannitol PEARLITOL® 200 SD (SDman), both from Roquette Frères (Lestrem, France); Roquette Magnesium Stearate from Roquette Frères (Lestrem, France); Sertraline: StarPharm (China).
Methods
Blending
Mannitol and sertraline were mixed for 5 min in a Turbula mixer (WAB-Group, Muttenz, Switzerland). Additional mixing for 5 min was done after adding the magnesium stearate.
Powder characteristics
Bulk density (BD) and tapped density (TD) were measured and Hausner ratio (HR) calculated as per European Pharmacopeia 2.9.34.
Powder flow time (FT) and angle of repose (AR) were measured as per European Pharmacopeia 2.9.16.
Tableting
All tableting trials were done on a high-speed rotary tablet press simulator: STYLCAM 200R (MEDELPHARM), using KORSCH XL400 profile.
Punches/Die: Euro B Elizabeth D10R10
Tablet weight: 400 mg
Lubrication: 1% magnesium stearate
Compression force: 5, 10, 15, 20 kN
Precompression force: 0 or 20% of the compression force
Simulated tableting speed: 85,000 tablets/hour.
Tablet characterization
All tablets were visually inspected; capped tablets are recorded without any further characterization. Per test, 10 tablets were tested for their dimensions, weight, and hardness, using a Pharmatron ST 50 (Solothurn, Switzerland) equipment.
RESULTS
The sertraline powder had very poor flow properties and was not usable on a rotary press. Indeed, it was not possible to produce any tablet on the compression simulator. Blends with SDman were free flowing up to 15% sertraline (see table 1). The blend with 20% sertraline is not free-flowing and to avoid inconsistent die feeding, a force feeder was used for all trials on a tablet press simulator.
Table 1. Powder characteristics of sertraline/SDman blends
|
Sertraline/SDman
|
Bulk density |
Tapped density
|
Hausner ratio |
Angle of repose
|
Flow time |
| 0/100 | 0.513 | 0.610 | 1.19 | 32 | 5 |
| 5/95 | 0.569 | 0.646 | 1.14 | 31 | 4 |
| 10/90 | 0.556 | 0.662 | 1.19 | 36 | 4 |
| 15/85 | 0.547 | 0.658 | 1.20 | 38 | 5 |
| 20/80 | 0.577 | 0.702 | 1.22 | 38 | infinite |
| 100/0 | 0.455 | 0.676 | 1.49 | 54 | infinite |
GTman showed better flow properties than SDman. All blends with sertraline and GTman were flowing freely.
Table 2. Powder characteristics of sertraline/GTman blends
| Sertraline/SDman mass ratio |
Bulk density |
Tapped density |
Hausner ratio |
Angle of repose |
Flow time
|
| 0/100 | 0.650 | 0.750 | 1.15 | 29 | 4 |
| 5/95 | 0.67 | 0.75 | 1.12 | 30 | 3 |
| 10/90 | 0.684 | 0.773 | 1.13 | 29 | 3 |
| 15/95 | 0.690 | 0.821 | 1.19 | 28 | 3 |
| 20/80 | 0.685 | 0.834 | 1.22 | 32 | 4 |
| 25/75 | 0.685 | 0.848 | 1.24 | 33 | 9 |
| 100/0 | 0.455 | 0.676 | 1.49 | 54 | infinite |
The tableting trials demonstrate the need of precompression when formulating a tablet of sertraline with SDman (see figure 1). Without precompression, capping occurs at 20 kN compression force for 5% and 10% sertraline and 15 kN compression force for 15% sertraline. In these conditions, 100 N tablet hardness is accessible with 5% and 10% and not with 15% of sertraline. Adding a precompression step, capping starts to appear at 15% sertraline (high standard deviation of the tablet hardness at 20 kN compression force), and tablets are not feasible with 20% sertraline. For a robust formulation maximizing API content, formulator will choose the formulation with 15% sertraline and use precompression
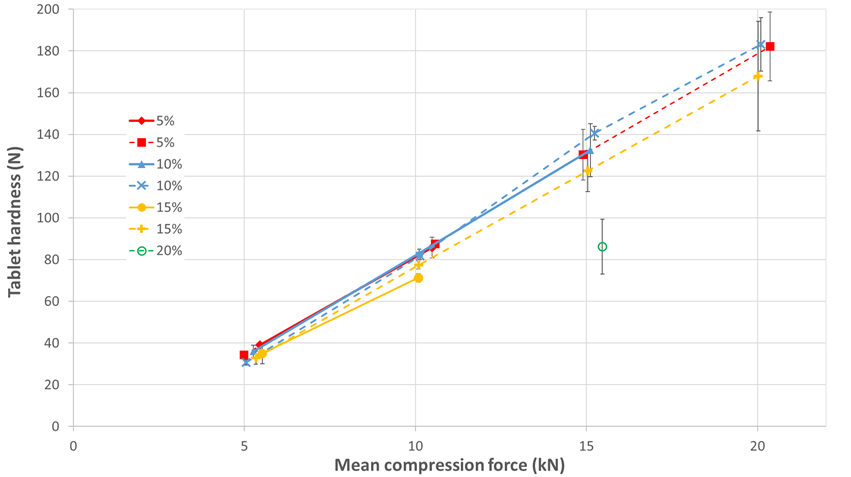
Figure 1. SDman; tablet hardness function of compression force with (dotted line) and without (solid line) precompression. Sertraline content is indicated in the legend.
The behavior of GTman was completely different. Without precompression, there was no capping observed till 25% of sertraline (see figure 2). This study confirms that this new directly compressible mannitol grade developed to improve tabletability is effective even when formulating tablets with a challenging API. Logically, the tablet hardness decreases with the sertraline ratio increase. Till 20% sertraline, 100 N tablet hardness is reachable at just 15 kN compression force while at 25% sertraline, a higher compression force is required.
At 15% sertraline, it is not possible to obtain tablets with SDman without precompression. The addition of a precompression step allows to obtain SDman/sertraline tablets with acceptable hardness (122 N for 15 kN). As GTman shows a better tabletability, the same hardness is easily accessible with GTman without precompression. Indeed, the tablet hardness is lower for GTman without precompression in comparison with SDman using precompression, but it remains conformed to the 100 N tablet hardness objective. With GTman, the higher tabletability and the absence of capping gives access to lower compression force and no precompression to reach a similar tablet quality.
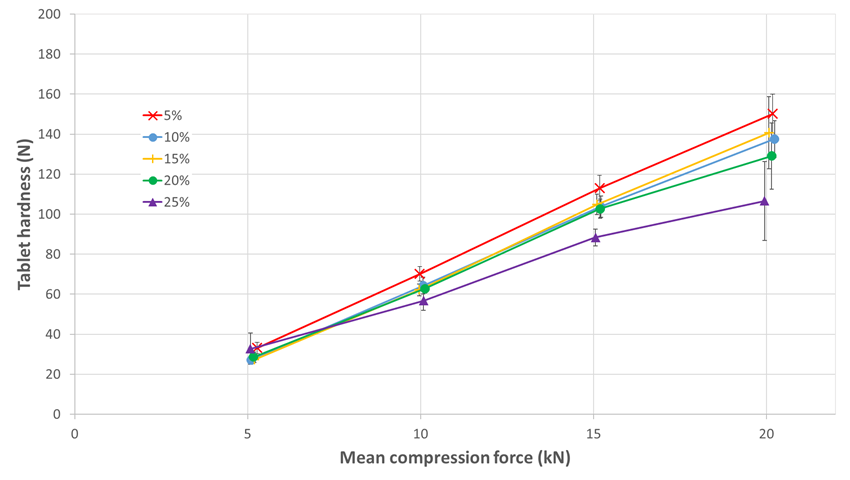
Figure 2. GTman; tablet hardness function of compression force at increasing sertraline ratio without precompression.
CONCLUSION
In this tableting study using sertraline as model API, the new granulated DC mannitol GTman, is very efficient in overcoming capping. A formulation that necessitates high compression force or precompression using spray dried DC mannitol (SDman) can thus be manufactured on an industrial scale without the need for these settings when utilizing GTman. That presents two major benefits for industrial tablets production:
- On a common high speed rotary press, the use of lower compression forces and/or no precompression decreases the probability of facing capping, reduces the wear of punches and prolongs their lifespan.
- On a high speed double rotary press, the tablet production output can be doubled leveraging the additional compression roller, not used for applying precompression.
REFERENCES
1. Boldhuis G.K., Rexwinkel E.G., Zuurman K., Polyols as filler-binders for disintegrating tablets prepared by direct compaction, Drug Dev. Ind. Pharm., 35,671-677 (2009)
https://doi.org/10.1080/03639040802587799
2. Tarlier N., Lefèvre P., Simon D., Characterization of different textured mannitol tabletability using a rotary tablet press simulator, Poster Compaction Simulation Forum, Ghent (2017)
3. Lefèvre, P., Haeusler, O., Boit, B., Croquet, S., Comparison of capping tendency of directly compressible mannitol powders, Poster, 4th European Conference on Pharmaceutics, 20-21 March 2023, Marseille, France
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.
The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission.