Polysorbates versus Hydroxypropyl beta Cyclodextrin: Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies
Published February 11, 2026
Results presented here are from Molecules 2022, 27, 6497 https://doi.org/10.3390/molecules27196497
Author(s)
Hailong Zhang
Analytical Manager, R&D analysis, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Shiqi HONG
Biopharma Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Sarah Si Kai Tan
Assistant Research Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Tao Peng
BioPharma Research Manager, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Lucas Yuan Hao Goh
Biopharma Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Kwan Hang Lam
Analytical Senior Scientist, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Keat Theng Chow
Pharmaceutical Research Manager, Pharmaceutical R&D, Roquette Asia Pacific Pte Ltd, 138588 Singapore
Rajeev GOKHALE
Head of Global Pharmaceutical Sciences, Roquette America Inc., Geneva, IL 60134, USA
Introduction
Polysorbates (PS 20 and PS 80) are the most widely used surfactants in biopharmaceutical formulations to protect proteins from denaturation, aggregation, and surface adsorption. To date, more than 90% of marketed therapeutic antibodies contain either PS 20 or PS 80 in their formulations.1 However, Polysorbates are chemically diverse mixtures, which are prone to degradation by oxidation and hydrolysis to produce peroxides and fatty acids which in turn, induce protein oxidation, aggregation, and insoluble particle formation.2,3 These will negatively impact protein quality and stability. Thus, polysorbate degradation has emerged as one of the major challenges in the development and commercialization of therapeutic protein products. Hydroxypropyl beta-cyclodextrin (HPβCD) has been shown as a new multifunctional excipient that provides protein stabilization functions in biopharmaceutical downstream processes and final formulation.4
This study aims to evaluate HPβCD, which is a well-established excipient used in many approved small molecule drug products, against polysorbates as a stabilizer in biologics formulations. In the study, the chemical stability of KLEPTOSE® HPβCD (KLEPTOSE® HP Biopharma grade, KLEPTOSE® HPB Biopharma grade, KLEPTOSE® HPB -LB Parenteral grade: differing by their molar substitution (MS) degree) was compared with polysorbates (PS 20 and PS 80) under various stress conditions. In addition, using monoclonal antibody adalimumab as a model protein, the physicochemical stability of the protein in formulations with PS 80 or KLEPTOSE® HPB Biopharma as stabilizers was investigated
Results And Discussion
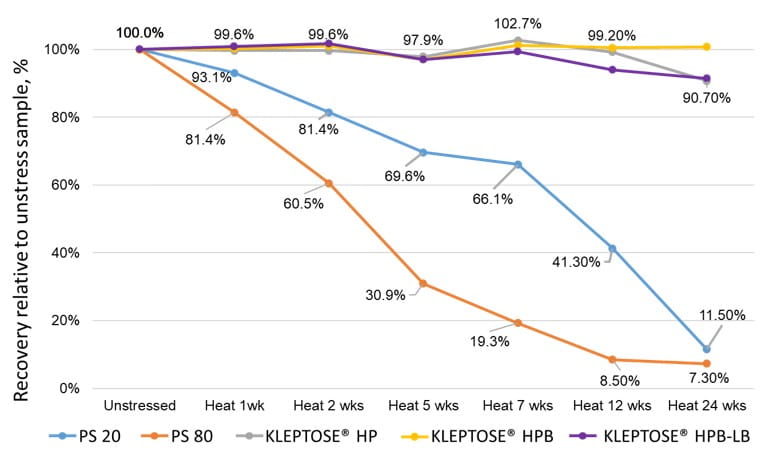
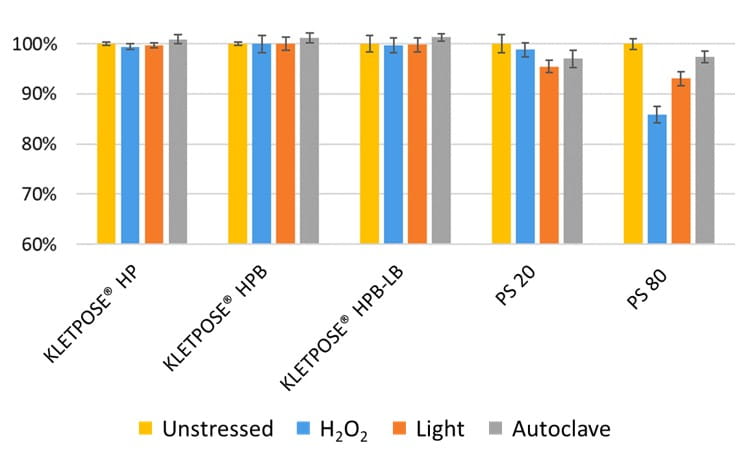
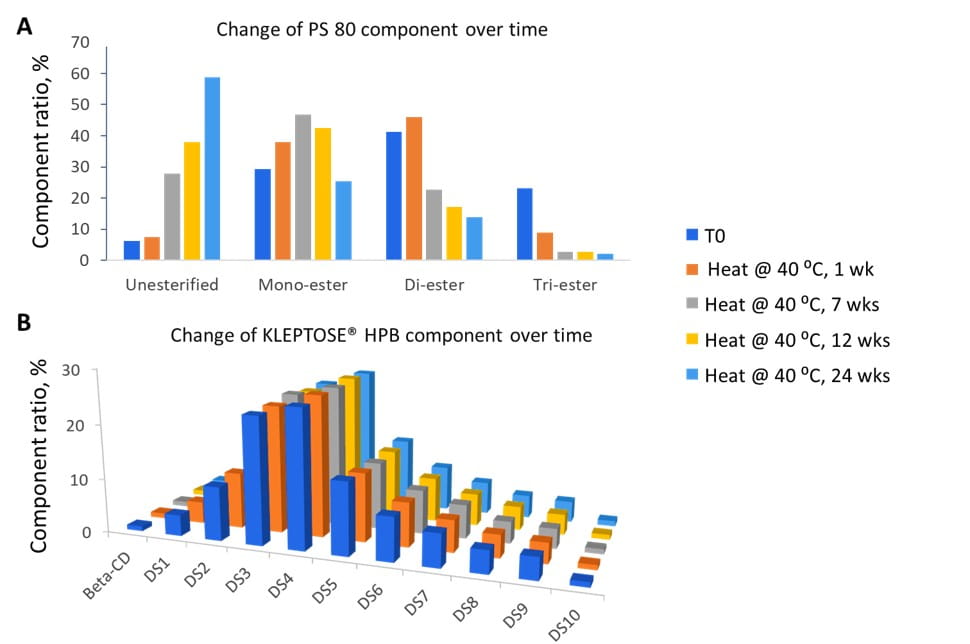
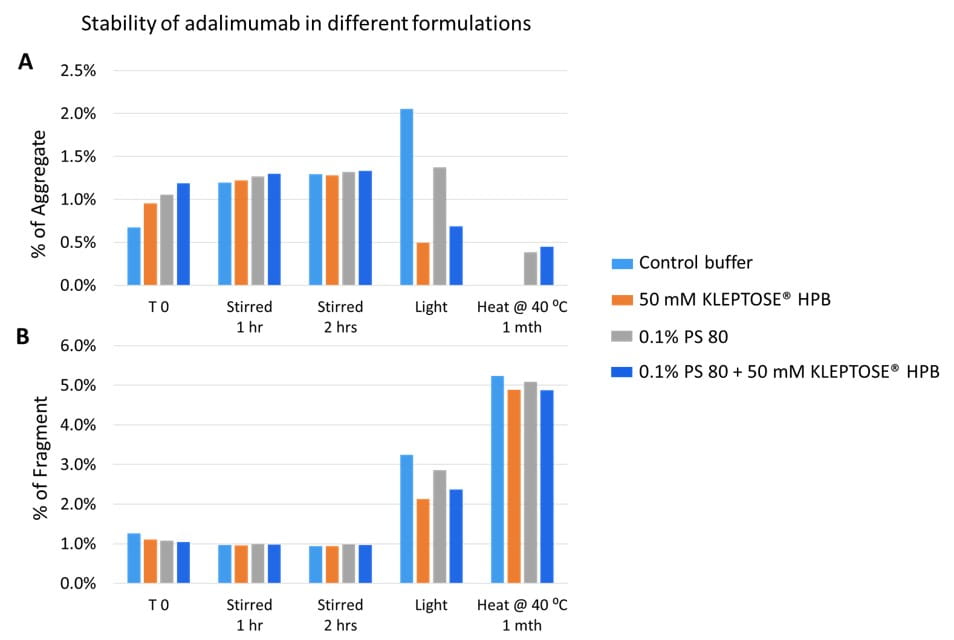
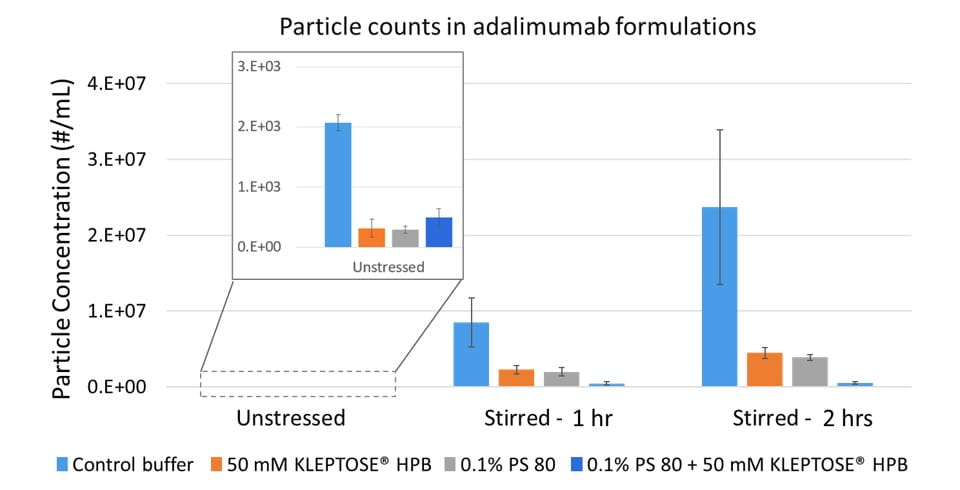
An accelerated stability study on polysorbates and KLEPTOSE® HPβCD has been conducted to compare the chemical stability under different stress conditions: mild thermal (40°C/75% RH), harsh thermal (autoclaved for 5 cycles at 121°C/15 min), light, and oxidative stresses (figure 1). When subjected to mild thermal stress at 40°C until week 24, KLEPTOSE® HPβCD showed slight changes in product recovery (90.7%-100.7% for different grades of HPβCD) while both PS 20 and PS 80 suffered significant degradation with the product recovery of 11.5% and 7.3%, respectively (figure 2). When subjected to autoclave cycles, light, and oxidative stresses, HPβCDs remained stable while polysorbates showed relatively more degradation with product recovery of 95.5% to 98.8% for PS 20, and 85.5% to 97.4% for PS 80 (figure 3). Profiling characterization and degradation analysis upon thermal stress revealed that the chemical structures of HPβCDs remained intact (figure 4A) while polysorbates underwent significant hydrolytic degradation and oxidation (figure 4B). Lastly, the physicochemical stability of monoclonal antibody in formulations containing PS 80 or KLEPTOSE® HPB have been investigated using adalimumab as a model protein (formulation details in table 1). When subjected to light stress, KLEPTOSE® HPB-containing formulations showed significantly lower protein aggregation, as well as superior monomer and total protein recovery compared to polysorbate-containing formulations (figure 5). Under heat stress at 40°C for 1 month, KLEPTOSE® HPB-containing formulations presented comparable protein stabilization function to PS 80-containing formulations. Adalimumab remained stable in all formulations under 2-hour agitation stress. Both KLEPTOSE® HPB and PS 80 had successfully reduced agitation-indued subvisible particle formation under agitation stress (figure 6). Interestingly, the formulation containing a mixture of KLEPTOSE® HPB and PS 80 demonstrated the lowest sub-visible particle counts indicating a possible synergistic effect of the stabilizer combination.
Conclusion
HPβCD has been applied in several approved parenteral small molecule drugs. In view of its high-water solubility and extremely stable structure, KLEPTOSE® HPβCD shows great promise as a multifunctional excipient to prevent protein aggregation, and to reduce sub-visible particle formation in biopharma downstream processes and formulations. KLEPTOSE® HPβCD can serve as an effective alternative functional excipient to polysorbate in therapeutic protein formulations.
Figure 1. Summarized stress conditions on polysorbates and KLEPTOSE® HPβCD.
Figure 2. Thermal stability of polysorbates and KLEPTOSE® HPβCD at 40°C/75% RH.
Figure 3. Comparative product recovery of KLEPTOSE® HP, HPB and HPB-LB, PS 20, and PS 80 under autoclave, oxidative and light stresses.
Figure 4. Molecular profiling of representative polysorbate - PS 80 (A) and representative HPβCD - KLEPTOSE® HPB (B) under thermal stress (40°C/75% RH) for up to 24 weeks.
Figure 5. Aggregation (A) and fragmentation (B) of adalimumab in different formulations under various stresses.
Figure 6. Comparison of sub-visible particles in adalimumab formulations under agitation stress.
Table 1. Adalimumab formulations for stress studies.
| SN | Name | Protein Concentration | Formulation |
| 1 | Control buffer | 5 mg/mL adalimumab | Phosphate/citrate, pH 5.2, 1.2% mannitol |
| 2 | 50 mM KLEPTOSE® HPB | Phosphate/citrate, pH 5.2, 1.2% mannitol, 50 mM KLEPTOSE® HPB | |
| 3 | 0.1% PS80 | Phosphate/citrate, pH 5.2, 1.2% mannitol, 0.1% PS80 | |
| 4 | 0.1% PS80 + 50 mM KLEPTOSE® HPB | Phosphate/citrate, pH 5.2, 1.2% mannitol, 50 mM KLEPTOSE® HPB, 0.1% PS80 |
References
1. Bollenbach, L.; Buske, J.; Mader, K.; Garidel, P., Poloxamer 188 as surfactant in biological formulations - An alternative for polysorbate 20/80? Int J Pharm 2022, 620, 121706.
https://doi.org/10.1016/j.ijpharm.2022.121706
2. Kerwin, B. A., Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci 2008, 97 (8), 2924-35.
https://doi.org/10.1002/jps.21190
3. Dixit, N.; Salamat-Miller, N.; Salinas, P. A.; Taylor, K. D.; Basu, S. K., Residual Host Cell Protein Promotes Polysorbate 20 Degradation in a Sulfatase Drug Product Leading to Free Fatty Acid Particles. J Pharm Sci 2016, 105 (5), 1657-1666.
https://doi.org/10.1016/j.xphs.2016.02.029
4. Serno, T.; Carpenter, J. F.; Randolph, T. W.; Winter, G., Inhibition of agitation-induced aggregation of an IgG-antibody by hydroxypropyl-beta-cyclodextrin. J Pharm Sci 2010, 99 (3), 1193-206.
https://doi.org/10.1002/jps.21931
® Registered trademark(s) of Roquette Frères. The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without any guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission. For questions about a product’s compliance with additional countries’ standards not listed above, please contact your local Roquette representative.