Accelerated Stability Study of Biopharma Grade Hydroxypropyl β-Cyclodextrin in Solution
Published March 07, 2026
Author : Joel Delattre
Senior Chemical Technician, Roquette R&D
Laetitia Hermoire
Senior Analytical Technician, Roquette R&D
Dany Duhamel
Senior Analytical Technician, Roquette R&D
Sandra Bigot
Chemical Engineer, Roquette
Pierre Heijboer
Previously Manager of the Structural Analysis Laboratory, Roquette R&D
Juliette Caron
Previously Analytical Scientist, Roquette R&D
1. Introduction
Hydroxypropyl β-cyclodextrins (HPβCD) are frequently used in biopharmaceutical applications to stabilize therapeutic proteins. Actually, HPβCD exhibit surface activity and anti-aggregation properties enabling the shelf-life extension of such proteins. In addition, HPβCD also reduce thermal and agitation sensitivity of drug formulations. However, the wide variety of conditions encountered by biopharmaceutical formulations, such as acidic or alkaline pH, ionic strength or the presence of oxygen, could lead to some degradation of HPβCD. The objective of the present study is to monitor HPβCD behavior, including its degradation, by existing quantitative analytical methods. Therefore, KLEPTOSE® HP Biopharma low endotoxin hydroxypropyl β-cyclodextrin (high molar substitution) and KLEPTOSE® HPB Biopharma low endotoxin hydroxypropyl β-cyclodextrin (low molar substitution) were subjected to accelerated stability study under specific stress conditions in aqueous solution.
Material and Methods
Materials
Two grades of HPβCD with different molecular substitution (MS) were used for this study: KLEPTOSE® HPB Biopharma and KLEPTOSE® HP Biopharma (respective nominal MS of 0.62 and 0.9).
Acidic, phosphate and ammonium salts were used as buffers.
Accelerated stability study performed under Petrotest
For accelerated degradation, 200 mM HPβCD solutions were placed in different conditions (various buffer concentrations, ionic strength and pH) under thermal stress (40°C for 12 hours) and oxidative atmosphere (initial oxygen pressure of 700 KPa) in a small-scale tester PetroOxy® (Anton Paar). The oxidation was measured by the percentage decrease of oxygen pressure (%PO2). This study was performed at different values of pH (3, 5, 7 and 9), ionic strength (0 mM and 150 mM) and buffer concentration (5 mM and 50 mM) as listed in table 1.
Table 1: Stability study experimental conditions
| Sample | Buffer concentration (mM) | pH | Ionic strength [NaCl] (mM) | PO2 decrease (%) | |
| KLEPTOSE® HPB | 1 | 5 | 3 | 0 | 0.4 |
| 2 | 5 | 3 | 150 | 1.3 | |
| 3 | 50 | 3 | 150 | 0.7 | |
| 4 | 5 | 5 | 0 | 0.5 | |
| 5 | 5 | 5 | 150 | 0.7 | |
| 6 | 5 | 7 | 0 | 0.4 | |
| 7 | 5 | 7 | 150 | 0.7 | |
| 8 | 5 | 9 | 0 | 0.7 | |
| 9 | 5 | 9 | 150 | 0.7 | |
| KLEPTOSE® HP | 10 | 5 | 3 | 0 | 0.8 |
| 11 | 5 | 3 | 150 | 1.7 | |
| 12 | 50 | 3 | 150 | 0.4 | |
| 13 | 5 | 5 | 0 | 0.9 | |
| 14 | 5 | 5 | 150 | 0.7 | |
| 15 | 5 | 7 | 0 | 0.7 | |
| 16 | 5 | 7 | 150 | 0.5 | |
| 17 | 5 | 9 | 0 | 0.7 | |
| 18 | 5 | 9 | 150 | 0.7 |
Analytical characterisation of HPβCD degradation
HPβCD degradation was estimated by measuring key structural parameters:
- Molar degree of substitution (MS) by H Nuclear Magnetic Resonance (NMR)1: measures the number of hydroxypropyl (HP) moieties per glucose unit (can be also calculated per β-CD unit).
- Reducing sugars content by the Somogyi-Neslon methodology: reflects HPβCD hydrolysis extent.
- Polydispersity fingerprint by ESI-MS (ElectroSpray Ionization-Mass Spectroscopy): reflects the diversity of substituted β-CD species and their respective stability. However, this technique does not give information about the number of isomers in one specific substituted family (i.e β-CD having 2 HP might have several position isomers.1 Polydispersity fingerprint gives a qualitative information about isomers.
Results and discussion
HPβCD degradation qualitative evaluation: polydispersity fingerprint by ESI-MS
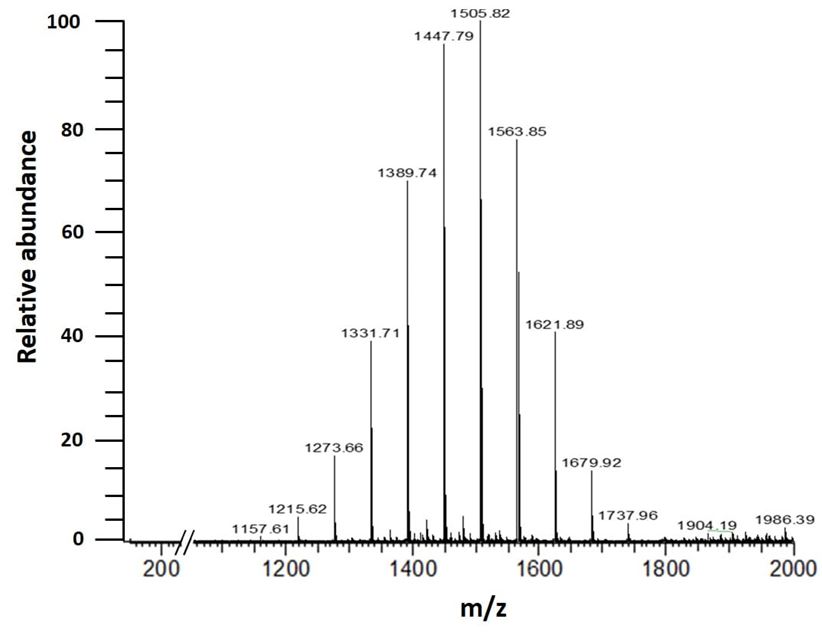
ESI-MS analysis is a widely used first-hand tool to qualitatively evaluate HPβCD polydispersity. Each substituted specie has a characteristic molecular weight (MW) detected by the mass spectrometer. Depending on MS, each HPβCD product has its specific specie distribution: the higher the MS, the higher the MW corresponding to a higher number of hydroxypropyl group (HP) on one β-cyclodextrin molecule (β-CD). A typical polydispersity fingerprint acquired by ESI-MS is given in Figure 1 with the intensity on the y-axis and the mass (m) on the charge (z) on the x-axis. For this technic, molecules are ionized by electrospray with sodium ion, so each detected mass m/z reflects one HPβCD specie ionized with a Na+ ion (Table 2).
Figure 1: KLEPTOSE® HPB Biopharma polydispersity fingerprint, 0,1 g/L w/w in 1 mM acetate sodium buffer – ESI-MS – positive mode.
Table 2. Different species that comprise HPCD, their molecular weight (MW) and corresponding m/z value [M+Na]+ detected by ESI-MS, positive mode. [M+Na]+ is here equal to the MW of the specie to which a sodium ion is added (molecular weight of sodium is about 23 g/mol). This table lists the molecules from non-substituted β-CD called HP0 to HPβCD with total substitution substituted with 15 HP groups (HP 15). The HP number therefore represents the number of hydroxy groups substituted by a hydroxypropyl group.
| Isomer of β-CD | MW (g/mol) | M+Na+ |
| β-CD = HP 0 | 1134 | 1157 |
| HP 1 | 1192 | 1215 |
| HP 2 | 1250 | 1273 |
| HP 3 | 1308 | 1331 |
| HP 4 | 1366 | 1389 |
| HP 5 | 1424 | 1447 |
| HP 6 | 1482 | 1505 |
| HP 7 | 1540 | 1563 |
| HP 8 | 1598 | 1621 |
| HP 9 | 1656 | 1679 |
| HP 10 | 1714 | 1737 |
| HP 11 | 1772 | 1795 |
| HP 12 | 1980 | 1853 |
| HP 13 | 1888 | 1911 |
| HP 14 | 1946 | 1969 |
| HP 15 | 2004 | 2027 |
The column M+Na+ represents the mass of the ionized compound that it means molecule mass (MW column) implemented from the mass of sodium (23 g/mol) used for ionization. This type of analysis gives a qualitative picture of HPβCD diversity. This can be a relevant indicator if degradation occurring during stability study. However, no interpretation related to peak intensity and species abundance can be drawn from such a spectrum.
Figure 2 compares ESI-MS fingerprint of KLEPTOSE® HP Biopharma (reference 2 in table 2) with three samples of KLEPTOSE® HP Biopharma subjected to the accelerated stability study in the Petrotest (samples 10, 11 and 13 in table 1).
Figure 2. Polydispersity fingerprint – ESI-MS – positive mode of KLEPTOSE® HP Biopharma (reference 2) and KLEPTOSE® HP Biopharma subjected to the accelerated stability study in the Petrotest (samples 10, 11 and 13 in table 1).
No significant differences are highlighted by ESI-MS analysis shown on figure 2. The species distribution of different HPβCD for KLEPTOSE® HP are the same before and after the stress conditions application.
HPβCD degradation quantitative approach: MS and Reducing sugars
HPβCD degradation can be measured following two quantitative parameters: the decrease of the molar substitution (MS) and the increase of the reducing sugars concentration. These two parameters were measured for all samples after storage in oxidative conditions as listed in table 1.
Table 3. Molecular Substitution (MS) and reducing sugars content measured for HPβCD after storage under different oxidative conditions. Reference 1 and 2 correspond to the analysis of initial KLEPTOSE® HPB and HP respectively before oxidation. Samples 1 to 18 are referring to table 1 and were stressed through oxidation at 700 kPa oxygen pressure in different conditions (pH, ionic strength and buffer concentration).
| Sample Name | MS | Reducing Sugars | |||
| KLEPTOSE® HPB | Reference 1 | 0.61 | < 50 | ||
| 1 | 0.6 | 50 | |||
| 2 | 0.61 | < 50 | |||
| 3 | 0.61 | < 50 | |||
| 4 | 0.6 | 90 | |||
| 5 | 0.6 | < 50 | |||
| 6 | 0.61 | < 50 | |||
| 7 | 0.6 | < 50 | |||
| 8 | 0.61 | < 50 | |||
| 9 | 0.6 | < 50 | |||
KLEPTOSE® HP | Reference 2 | 0.87 | 80 | ||
| 10 | 0.87 | < 50 | |||
| 11 | 0.87 | < 50 | |||
| 12 | 0.87 | < 50 | |||
| 13 | 0.87 | < 50 | |||
| 14 | 0.87 | < 50 | |||
| 15 | 0.87 | < 50 | |||
| 16 | 0.87 | < 50 | |||
| 17 | 0.86 | < 50 | |||
| 18 | 0.86 | < 50 | |||
It appears clearly in table 3 that the molecular substitution of both KLEPTOSE® HPB and HP does not vary, whatever the storage conditions. In fact, measured MS values after 12h at 40°C under high oxidative conditions (700 kPa PO2) remain identical to the MS value of the same HPβCD before oxidation (reference 1 and 2 respectively), no matter the composition of the solution.
Regarding the reducing sugars concentration, a similar behavior is observed. Slight variations of a few tens of ppm could be noticed between the reference samples and the ones after storage. However, those changes are not occurring in a consistent way (for example: increase between reference 1 and sample 4 and decrease between reference 2 and samples 10 to 18), showing that they are part of the measurement uncertainty.
5. Conclusions
The aim of this study was to evaluate the stability of two biopharma grades of HPβCD products having different MS values (KLEPTOSE® HP Biopharma = 1501 g/mol and KLEPTOSE® HPB Biopharma = 1387 g/mol). It is demonstrated that pH and ionic strength have insignificant effect on polydispersity fingerprint, MS and reducing sugar content as per figure 2 and table 3. KLEPTOSE® HPB Biopharma and KLEPTOSE® HP Biopharma in solution are thus stable under stress conditions (40°C, 700 kPa O2 pressure, 150 mM ionic strength, at pH 3, 5, 7 and 9).
References
1. Pitha, J.; Szabo, L.; Fales, H.M. Reaction of cyclodextrins with propylene oxide or with glycidol: Analysis of product distribution, Carbohydrate Research, 1987, 168, 191-198
https://doi.org/10.1016/0008-6215(87)80025-3
® Registered trademark(s) of Roquette Frères.
The information contained in this document is to the best of our knowledge true and accurate, but all instructions, recommendations or suggestions are made without any guarantee. Since the conditions of use are beyond our control, we disclaim any liability for loss and/or damage suffered from use of these data or suggestions. Furthermore, no liability is accepted if use of any product in accordance with these data or suggestions infringes any patent. No part of this document may be reproduced by any process without our prior written permission. For questions about a product’s compliance with additional countries’ standards not listed above, please contact your local Roquette representative.