
Roquette LYCOAT® ReadiLYCOAT®, the outstanding coating solution
Is a standard coating solution only just good enough for you?
Discover our LYCOAT® ReadiLYCOAT®, a natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
LYCOAT® Key benefits
Natural origin, no solvent
The origin of LYCOAT® polymers is natural, vegetal and renewable. Obtained from the pea, the LYCOAT® manufacturing process uses water as the sole solvent. LYCOAT® RS 720 is the medium viscosity grade, LYCOAT® RS 780 is the low viscosity grade.
Superior organoleptic properties.
Compared to synthetic or highly modified polymers, LYCOAT® has a pleasant neutral odour and taste
| TEST 1 | TEST 2 | |||
| Preference | HPMC | LYCOAT | PVA | LYCOAT |
| Odour (n=15) | 20% | 80% | 7% | 93% |
| Taste (n=14) | 14% | 86% | 0% | 100% |
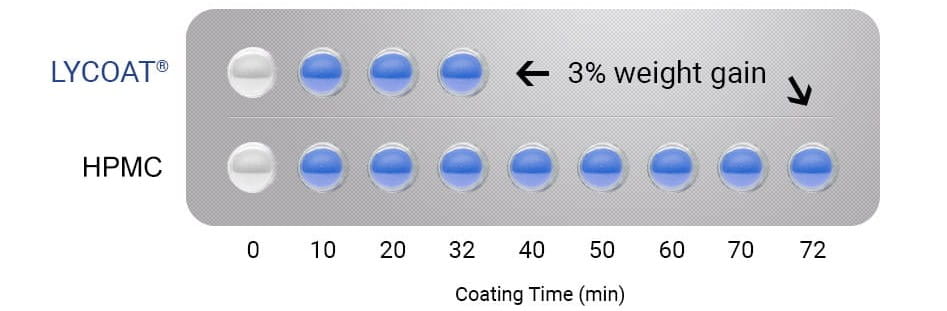
Quick coating
High solids content coating suspensions allow you to save up to 50% of coating time.
Fast and easy dispersion
Inertness
ReadiLycoat® key benefits
- ReadiLYCOAT® coating systems are designed to facilitate your coating operations and composition can be adapted to each of your needs in compliance with regulations.
- ReadiLYCOAT® improves the appearance of your tablets, capsules and granules.
- ReadiLYCOAT® MS also provides protection against moisture.
Expertise at your service
On-site assistance
Assistance for your trials
Process parameters optimization
Technical assistance
Development of «in-house» formulation
Feasibility trials: send us your tablet
Troubleshooting solutions
Colour matching solutions
Contact us for more information
On-site assistance (trials, process optimization)
Feasibility trial (send us your tablet)
Troubleshooting
Develop an «in house» coating formulation
Colour matching (ready mix coating system)
Sample request
Or any other subject, etc.
Resources
-
![A scientist observes through a microscope in a pharmaceutical lab.]() Safety – assured. Explore the story of our rigorous nitrosamine testing program, and how we help drug manufacturers navigate a shifting regulatory landscape.
Safety – assured. Explore the story of our rigorous nitrosamine testing program, and how we help drug manufacturers navigate a shifting regulatory landscape. -
![Pharmaceutical tablets lie in the center of a circle of water droplets]() Discover our portfolio of solutions for moisture sensitive active pharmaceutical and nutraceutical ingredients.
Discover our portfolio of solutions for moisture sensitive active pharmaceutical and nutraceutical ingredients. -
![A scientist observing through a microscope]() We provide our partners and their customers with unwavering quality standards, as well as a clear and secure supply chain that spans from the sourcing of our raw materials to the delivery of our finished products.
We provide our partners and their customers with unwavering quality standards, as well as a clear and secure supply chain that spans from the sourcing of our raw materials to the delivery of our finished products. -
![Analytical scientist working in a laboratory]() At our Asia Pacific innovation center in Singapore, we have a suite of analytical testing capabilities that are crucial to drug product development.
At our Asia Pacific innovation center in Singapore, we have a suite of analytical testing capabilities that are crucial to drug product development. -
![A family, including children, parents and grandparents, laugh and play on a beach.]() Explore our full range of excipients, raw materials and actives for pharmaceuticals, nutraceuticals, over-the-counter products, biopharmaceuticals, injectables and dialysis solutions.
Explore our full range of excipients, raw materials and actives for pharmaceuticals, nutraceuticals, over-the-counter products, biopharmaceuticals, injectables and dialysis solutions. -
![Close up of a young woman with brown hair smiling as she takes a white tablet.]() Discover what makes PEARLITOL® SD mannitol the industry’s first-choice excipient for direct compression, and how it can help you unlock advanced tablet dosage forms.
Discover what makes PEARLITOL® SD mannitol the industry’s first-choice excipient for direct compression, and how it can help you unlock advanced tablet dosage forms. -
![A little girl with pigtails and a pink striped t-shirt has an orodispersible tablet on her tongue]() Pharmaceutical and nutraceutical producers need more than just an ingredient supplier. Discover how our decades of expertise can help you overcome the toughest formulation challenges.
Pharmaceutical and nutraceutical producers need more than just an ingredient supplier. Discover how our decades of expertise can help you overcome the toughest formulation challenges. -
![Young girl holds clear pharmaceutical capsule between her teeth.]() The key ingredient behind an all-new vegetarian softgel technology: Pea starch is unlocking its potential as a powerhouse in pharmaceutical and nutraceutical applications.
The key ingredient behind an all-new vegetarian softgel technology: Pea starch is unlocking its potential as a powerhouse in pharmaceutical and nutraceutical applications. -
![]() How Much Can We Benefit from Working on a Common Sustainable Agricultural Approach?
How Much Can We Benefit from Working on a Common Sustainable Agricultural Approach? -
![A family walking in the forest]() Life-saving pharmaceuticals start with high-quality ingredients. Learn more about our premium solutions and how they can support your drug development projects.
Life-saving pharmaceuticals start with high-quality ingredients. Learn more about our premium solutions and how they can support your drug development projects. -
![A family shown in silhouette in front of the setting sun.]() Life-saving pharmaceuticals start with high-quality ingredients. Discover how we helped expand the potential of process China’s DMF.
Life-saving pharmaceuticals start with high-quality ingredients. Discover how we helped expand the potential of process China’s DMF. -
![A nurse wearing a green gown and cap prepares an IV drip]() Read all about our multi-compendial excipient for efficient drug delivery, and the benefits it can offer your injectable products.
Read all about our multi-compendial excipient for efficient drug delivery, and the benefits it can offer your injectable products. -
![Cusmotizing appealing gluten-free dosage forms]() PEARLITOL® SW-F (wheat-free) mannitol for gluten-free nutraceutical dosage forms
PEARLITOL® SW-F (wheat-free) mannitol for gluten-free nutraceutical dosage forms -
![An older couple holds up a bottle of tablets.]() Supplements? We’re here to support you. Get the facts on our full-stack formulation solutions for health-boosting nutraceuticals and OTC consumer products.
Supplements? We’re here to support you. Get the facts on our full-stack formulation solutions for health-boosting nutraceuticals and OTC consumer products. -
![]() Unlock effective drug manufacturing and enhance patient compliance with our extensive range of reliable fillers and filler-binders.
Unlock effective drug manufacturing and enhance patient compliance with our extensive range of reliable fillers and filler-binders. -
![]() Designed with functionality and flexibility in mind, our binders create robust formulations – no matter the application.
Designed with functionality and flexibility in mind, our binders create robust formulations – no matter the application. -
![A nurse wearing blue gloves prepares an IV bag]() Trust – it’s the most essential ingredient. Learn why hundreds of pharmaceutical manufacturers trust Roquette for its injectables and dialysis solutions.
Trust – it’s the most essential ingredient. Learn why hundreds of pharmaceutical manufacturers trust Roquette for its injectables and dialysis solutions. -
![Woman taking a pink tablet]() Rely on Roquette to help improve consumer compliance. Discover the key to formulating stable, easy-to-administer Oral Disintegrating Tablets (ODTs) with our quality excipients.
Rely on Roquette to help improve consumer compliance. Discover the key to formulating stable, easy-to-administer Oral Disintegrating Tablets (ODTs) with our quality excipients. -
![Close up of a child holding a white tablet on an outstretched tongue.]() An ageing global population, demand for more convenient dosage forms and the need to improve patient compliance are driving the development of new pathways for orally disintegrating dosage forms.
An ageing global population, demand for more convenient dosage forms and the need to improve patient compliance are driving the development of new pathways for orally disintegrating dosage forms. -
![Roquette back from Vitafood 2018]() Customizing oral delivery forms - the healthy way!
Customizing oral delivery forms - the healthy way! -
![A woman with brown hair, wearing a white coat, checking a machine in a pharmaceutical laboratory]() Don’t let poor bioavailability hold you back. Enhance your pharmaceutical and nutraceutical oral dosage forms with our versatile solubilizing solutions.
Don’t let poor bioavailability hold you back. Enhance your pharmaceutical and nutraceutical oral dosage forms with our versatile solubilizing solutions. -
![Leaflet disintegrants and superdisintegrants leaflet]() A consistent disintegration and dissolution profile is critical for the delivery of your medications’ active ingredients. Discover our solutions.
A consistent disintegration and dissolution profile is critical for the delivery of your medications’ active ingredients. Discover our solutions. -
![tablets]() A superdisintegrant that has excellent performance and cost-benefits.
A superdisintegrant that has excellent performance and cost-benefits. -
![tablets towel]() A highly efficient superdisintegrant with high efficiency even when used in lower doses.
A highly efficient superdisintegrant with high efficiency even when used in lower doses. -
![tablets gelules]() A partially depolymerized cellulose widely used in direct compression or in dry and wet granulation process.
A partially depolymerized cellulose widely used in direct compression or in dry and wet granulation process. -
![Tabulose]() Get to know our leading co-processed suspension agent, specifically developed to enhance semi-solid formulas.
Get to know our leading co-processed suspension agent, specifically developed to enhance semi-solid formulas. -
PEARLITOL® Flash, mannitol and starch compound, offers inert stability, fast disintegration and superior organoleptic properties for ODT formulations.
-
ReadiLYCOAT® is a breakthrough in film-coating for heat-sensitive products, offering a practical solution for tablet film-coating at low bed temperature.
-
![Close up of a child holding a white tablet on an outstretched tongue.]() KLEPTOSE® Linecaps pea maltodextrin allows the delivery of an efficient taste masking solution with the total safety offered by a widely used food ingredient, manufactured to the critical quality standards of pharmaceutical excipient.
KLEPTOSE® Linecaps pea maltodextrin allows the delivery of an efficient taste masking solution with the total safety offered by a widely used food ingredient, manufactured to the critical quality standards of pharmaceutical excipient. -
This study presents data supporting the superior compatibility of LYCOAT® in aqueous film coating of tablets with polyphenolic actives.
-
Learn more about Orally disintegrating tablets (ODTs), also known as orodispersible tablets, unique dosage forms formulated to improve their in vivo disintegration and dissolution rates.
-
![Young girl with brown hair holds white pill on outstretched tongue.]() PEARLITOL® is a unique combination, between mannitol and starch, which will allow you to formulate orodispersible tablets, easy to use and which disintegrate very quickly in the mouth.
PEARLITOL® is a unique combination, between mannitol and starch, which will allow you to formulate orodispersible tablets, easy to use and which disintegrate very quickly in the mouth. -
![]() The research press for the most demanding laboratories Simple precise data acquisition Clean, contained Rotary simulation: predicts production Simple to handly Small batches production
The research press for the most demanding laboratories Simple precise data acquisition Clean, contained Rotary simulation: predicts production Simple to handly Small batches production -
![Lycoat Readilycoat film coating]() Get to know our dream team for superior tablet coating: a natural inert polymer and ready-to-use coating system for fast aqueous film coating.
Get to know our dream team for superior tablet coating: a natural inert polymer and ready-to-use coating system for fast aqueous film coating. -
![Orally Disintegrating Tablets (ODTs)]() Overcoming your main challenges: disintegration time, stability, mouthfeel.
Overcoming your main challenges: disintegration time, stability, mouthfeel. -
This study has been conducted to evaluate the advantages of a specially pregelatinized, new-generation, hydroxypropyl starch polymer (LYCOAT®), over HPMC in aqueous film coating.
-
The main objective of this project was to investigate the potential of Kleptose Linecaps DE17 (pea maltodextrin with DE 15–20) in masking the bitter taste of antifungal drug, griseofulvin (GRI).
-
The study evaluates the taste masking performance of two new maltodextrins with high amylose content on NSAIDs.
-
This study was conducted to compare a non-GMO pregelatinized hydroxypropyl pea starch (LYCOAT® RS780) with HP corn and HP potato starch, as an alternative to gelatin in soft gel capsules.
-
This study has been done to compare the performance of LYCATAB® C with the similar excipient Starch 1500® in its recommended application as filler for two-piece hard gelatin capsules.
-
The aim of this study is to characterize both the pure substances and the physical mixtures of spray-dried lactose and maize starch in comparison to the new direct compression excipient StarLac®.
-
The aim of this study was to evaluate the coating quality of a modified pea starch based polymer in a ready-to-use coating formulation (ReadiLycoat®) at a process bed temperature lower than 25°C.
-
This study evaluates the potential of a new cold water soluble material with higher amount of amylose (KLEPTOSE® linecaps) to solubilise drugs.
-
The study assesses the suitability of excipients and superdisintegrants for developing an ODT platform using conventional and non-conventional tests.
-
The purpose of the study is to compare the powder and tablet characteristics of this new compound vs. the physical blend based on lactose and starch.
-
This poster presents Orally Disintegrating Films (ODF) as vectors for micro/nanoparticle delivery using Benzocaine as a model drug.
-
This study shows that the disintegration efficiency of sodium starch glycolate (SSG) is nearly independent from possible hydrophobic films on its surface.
-
Case study: Influence of Beta-Cyclodextrin Side Chain Substitutions on a Model BCS Class II CompoundThis poster studies the influence of beta-cyclodextrin side chain substitutions on the complexation efficiency of a model BCS Class II compound.
-
The objective of this study was to evaluate the impact of Magnesium stearate (MgSt) mixing conditions on the compaction behavior and disintegration time of different mannitol based formulations.
-
This poster presents an evaluation of LYCOAT® RS720 and its advantages over the Oral Disintegrating Film market.
-
This poster presents a new method for measuring the tablet disintegration swelling speed for a better understanding of the action of disintegrants.
-
The objective of this study was to evaluate the ability of native and modified β-cyclodextrins to enhance AMP solubility and stability.
-
The main objective of this study was to investigate the potential of KLEPTOSE® Linecaps DE17 (a pea maltodextrin) in masking the bitter taste of Caffeine Anhydrous (CA) by Hot Melt Extrusion (HME).
-
The aim of this study is to study the tablet formation of a compound based on lactose and starch (85:15 w/w) compared to the pure substances and graded physical mixtures. Pressure-time-profiles, pressure-porosity profiles and compactibility-plots help to evaluate the tableting properties.










































