PEARLITOL® Flash
Co-processed Mannitol Starch
EP/USP/JP compliance
|
Product Name |
PEARLITOL® Flash Co-processed Mannitol Starch |
||||||||
|
Synonyms |
D-Mannitol |
||||||||
|
Quantitative Composition |
Approximately D-Mannitol 80%-Extra White Maize Starch 20% |
||||||||
|
CAS Number |
D-Mannitol 69-65-8; Maize Starch 9005-25-8 |
||||||||
|
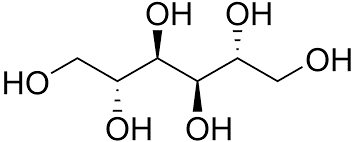
Chemical Structure |
D-Mannitol
|
||||||||
|
Molecular Weight |
Mannitol: 182.17 g/mol |
||||||||
|
Physical Form or Appearence |
White powder |
||||||||
|
Application |
PEARLITOL® Flash is a direct compression excipient with disintegrant properties. It offers excellent chemical inertness and consistent rapid disintegration time. It brings a pleasant taste and texture and is suitable for swallowable and orally dispersible tablets. |
||||||||
|
Water Content (LOD) |
Maximum water content: 3 % |
||||||||
|
Morphology |

|
||||||||
|
Average Mean Particle Diameter |
200 µm |
||||||||
|
Particle Size Distribution by Laser Diffraction |
dv10 = 80 µm dv50 = 200 µm dv90 = 300 µm |
||||||||
|
Solubility |
Partially soluble in cold water, practically insoluble in ethanol (96%) |
||||||||
|
Melting Temperature |
166-170°C for D-Mannitol |
||||||||
|
Water sorption isotherm at 20°C |

|
||||||||
|
Taste/Odor |
Slightly sweet (sweetening power of mannitol about 70% that of sucrose) |
||||||||
|
Compression Behaviour |

|
||||||||
|
Experimental Conditions for Compression Behavior |
|
||||||||
|
Powder Characteristics |
Powder Flowability: 5 s (according to Ph.Eur. 2.9.16, 10mm outflow opening) |
Disclaimer
® Registered trademark(s) of Roquette Frères. Any information provided herein is intended for healthcare and food industry professionals for internal use only and not to be delivered as such to final consumers. Information is based on our current state of knowledge and made available on an informational basis; products described may have restrictions with respect to their use, communication, and/or usage levels, and such may vary on a country-by-country basis. Manufacturers of dietary supplements should evaluate the intended use of the particular ingredient in their finished dietary supplement to confirm compliance with the applicable laws and regulations of authorities regulating such products, because the suitability and regulatory status of a product may be dependent on its specific intended use. As the use of these products is beyond our control, Roquette makes no express or implied warranties regarding the use of the product and no guarantee of product properties, and in particular no express or implied warranties regarding the use of the product in dietary supplements, including without limitation the implied warranties of merchantability and fitness for a particular purpose, and Roquette disclaims liability for any loss and/or damage related to such use. Roquette, further, does not warrant that the information or its use will not infringe any patent or other proprietary rights of any third party. Roquette providing this information is not a commitment to sell any product encompassing any of such information in the future.
 Starch
Starch